G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure
- PMID: 18635825
- PMCID: PMC2679955
- DOI: 10.1161/CIRCRESAHA.107.168336
G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure
Abstract
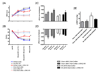
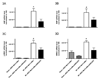
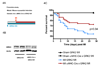
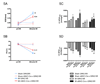
Myocardial G protein-coupled receptor kinase (GRK)2 is a critical regulator of cardiac beta-adrenergic receptor (betaAR) signaling and cardiac function. Its upregulation in heart failure may further depress cardiac function and contribute to mortality in this syndrome. Preventing GRK2 translocation to activated betaAR with a GRK2-derived peptide that binds G(beta)gamma (betaARKct) has benefited some models of heart failure, but the precise mechanism is uncertain, because GRK2 is still present and betaARKct has other potential effects. We generated mice in which cardiac myocyte GRK2 expression was normal during embryonic development but was ablated after birth (alphaMHC-Cre x GRK2 fl/fl) or only after administration of tamoxifen (alphaMHC-MerCreMer x GRK2 fl/fl) and examined the consequences of GRK2 ablation before and after surgical coronary artery ligation on cardiac adaptation after myocardial infarction. Absence of GRK2 before coronary artery ligation prevented maladaptive postinfarction remodeling and preserved betaAR responsiveness. Strikingly, GRK2 ablation initiated 10 days after infarction increased survival, enhanced cardiac contractile performance, and halted ventricular remodeling. These results demonstrate a specific causal role for GRK2 in postinfarction cardiac remodeling and heart failure and support therapeutic approaches of targeting GRK2 or restoring betaAR signaling by other means to improve outcomes in heart failure.
Figures



Similar articles
-
Gβγ-independent recruitment of G-protein coupled receptor kinase 2 drives tumor necrosis factor α-induced cardiac β-adrenergic receptor dysfunction.Circulation. 2013 Jul 23;128(4):377-87. doi: 10.1161/CIRCULATIONAHA.113.003183. Epub 2013 Jun 19. Circulation. 2013. PMID: 23785004 Free PMC article.
-
Gi-biased β2AR signaling links GRK2 upregulation to heart failure.Circ Res. 2012 Jan 20;110(2):265-74. doi: 10.1161/CIRCRESAHA.111.253260. Epub 2011 Dec 15. Circ Res. 2012. PMID: 22179058 Free PMC article.
-
Cardiac hyporesponsiveness in severe sepsis is associated with nitric oxide-dependent activation of G protein receptor kinase.Am J Physiol Heart Circ Physiol. 2017 Jul 1;313(1):H149-H163. doi: 10.1152/ajpheart.00052.2016. Epub 2017 May 19. Am J Physiol Heart Circ Physiol. 2017. PMID: 28526706
-
GRK2 inhibition in heart failure: something old, something new.Curr Pharm Des. 2012;18(2):186-91. doi: 10.2174/138161212799040510. Curr Pharm Des. 2012. PMID: 22229578 Review.
-
GRK2 as a novel gene therapy target in heart failure.J Mol Cell Cardiol. 2011 May;50(5):785-92. doi: 10.1016/j.yjmcc.2010.08.014. Epub 2010 Aug 25. J Mol Cell Cardiol. 2011. PMID: 20800067 Free PMC article. Review.
Cited by
-
Paroxetine-mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction.Sci Transl Med. 2015 Mar 4;7(277):277ra31. doi: 10.1126/scitranslmed.aaa0154. Sci Transl Med. 2015. PMID: 25739765 Free PMC article.
-
Cardiac G-protein-coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction.Circulation. 2012 May 1;125(17):2108-18. doi: 10.1161/CIRCULATIONAHA.111.044255. Epub 2012 Apr 10. Circulation. 2012. PMID: 22496128 Free PMC article.
-
Unheralded Adrenergic Receptor Signaling in Cellular Oxidative Stress and Death.Curr Opin Physiol. 2024 Aug;40:100766. doi: 10.1016/j.cophys.2024.100766. Epub 2024 Jun 24. Curr Opin Physiol. 2024. PMID: 39070968
-
The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits.Br J Pharmacol. 2010 Jul;160(6):1408-16. doi: 10.1111/j.1476-5381.2010.00793.x. Br J Pharmacol. 2010. PMID: 20590631 Free PMC article.
-
Targeting cardiac β-adrenergic signaling via GRK2 inhibition for heart failure therapy.Front Physiol. 2013 Sep 26;4:264. doi: 10.3389/fphys.2013.00264. Front Physiol. 2013. PMID: 24133451 Free PMC article. Review.
References
-
- Kudej RK, Iwase M, Uechi M, Vatner DE, Oka N, Ishikawa Y, Shannon RP, Bishop SP, Vatner SF. Effects of chronic beta-adrenergic receptor stimulation in mice. Journal of molecular and cellular cardiology. 1997;29(10):2735–2746. - PubMed
-
- Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, Yatani A, Dorn GW., 2nd Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101(14):1707–1714. - PubMed
-
- Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, Wight DC, Wagner TE, Ishikawa Y, Homcy CJ, Vatner SF. Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression. Circulation research. 1996;78(4):517–524. - PubMed
-
- Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB. Beta 1-adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nature medicine. 2003;9(10):1300–1305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL088243/HL/NHLBI NIH HHS/United States
- R01 HL056205-08/HL/NHLBI NIH HHS/United States
- R01 HL088243-01/HL/NHLBI NIH HHS/United States
- R01 HL061690-09/HL/NHLBI NIH HHS/United States
- R01 HL61690/HL/NHLBI NIH HHS/United States
- P01 HL075443/HL/NHLBI NIH HHS/United States
- R01 HL061690/HL/NHLBI NIH HHS/United States
- R01 HL087871/HL/NHLBI NIH HHS/United States
- P01 HL075443-050002/HL/NHLBI NIH HHS/United States
- R01 HL56205/HL/NHLBI NIH HHS/United States
- R01 HL87871/HL/NHLBI NIH HHS/United States
- R01 HL088243-02/HL/NHLBI NIH HHS/United States
- R01 HL056205/HL/NHLBI NIH HHS/United States
- R01 HL061690-11/HL/NHLBI NIH HHS/United States
- R01 HL061690-10/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

