Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation
- PMID: 18628995
- PMCID: PMC2442875
- DOI: 10.1371/journal.pone.0002703
Modulation of NKT cell development by B7-CD28 interaction: an expanding horizon for costimulation
Abstract
It has been demonstrated that the development of NKT cells requires CD1d. The contribution of costimulatory molecules in this process has not been studied. Here we show that in mice with targeted mutations of B7-1/2 and CD28, the TCRbeta(+)alpha-Galcer/CD1d(+) (iValpha14 NKT) subset is significantly reduced in the thymus, spleen and liver. This is mainly due to decreased cell proliferation; although increased cell death in the thymi of CD28-deficient mice was also observed. Moreover, in the B7-1/2- and CD28-deficient mice, we found a decreased percentage of the CD4(-)NK1.1(+) subset and a correspondingly increased portion of the CD4(+)NK1.1(-) subset. In addition, the mice with a targeted mutation of either B7 or CD28 had a reduced susceptibility to Con A induced hepatitis, which is known to be mediated by NKT cells. Our results demonstrate that the development, maturation and function of NKT cell are modulated by the costimulatory pathway and thus expand the horizon of costimulation into NKT, which is widely viewed as a bridge between innate and adaptive immunity. As such, costimulation may modulate all major branches of cell-mediated immunity, including T cells, NK cells and NKT cells.
Conflict of interest statement
Figures



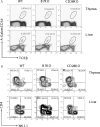
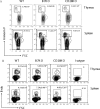

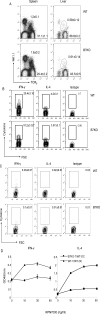
Similar articles
-
Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway.J Immunol. 2008 Jul 15;181(2):907-17. doi: 10.4049/jimmunol.181.2.907. J Immunol. 2008. PMID: 18606642 Free PMC article.
-
Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways.J Immunol. 2001 May 15;166(10):6012-8. doi: 10.4049/jimmunol.166.10.6012. J Immunol. 2001. PMID: 11342617
-
Dendritic cell maturation overrules H-2D-mediated natural killer T (NKT) cell inhibition: critical role for B7 in CD1d-dependent NKT cell interferon gamma production.J Exp Med. 2001 Oct 15;194(8):1179-86. doi: 10.1084/jem.194.8.1179. J Exp Med. 2001. PMID: 11602646 Free PMC article.
-
The role of B7 costimulation in T-cell immunity.Immunol Cell Biol. 1999 Aug;77(4):304-11. doi: 10.1046/j.1440-1711.1999.00835.x. Immunol Cell Biol. 1999. PMID: 10457196 Review.
-
CD28/B7 costimulation: a review.Crit Rev Immunol. 1998;18(5):389-418. doi: 10.1615/critrevimmunol.v18.i5.10. Crit Rev Immunol. 1998. PMID: 9784967 Review.
Cited by
-
Targeting NKT cells and PD-L1 pathway results in augmented anti-tumor responses in a melanoma model.Cancer Immunol Immunother. 2011 Apr;60(4):547-58. doi: 10.1007/s00262-010-0963-5. Epub 2011 Jan 15. Cancer Immunol Immunother. 2011. PMID: 21240487 Free PMC article.
-
Alternative memory in the CD8 T cell lineage.Trends Immunol. 2011 Feb;32(2):50-6. doi: 10.1016/j.it.2010.12.004. Epub 2011 Feb 1. Trends Immunol. 2011. PMID: 21288770 Free PMC article. Review.
-
Thymic resident NKT cell subsets show differential requirements for CD28 co-stimulation during antigenic activation.Sci Rep. 2020 May 19;10(1):8218. doi: 10.1038/s41598-020-65129-3. Sci Rep. 2020. PMID: 32427927 Free PMC article.
-
The Wiskott-Aldrich syndrome protein is required for iNKT cell maturation and function.J Exp Med. 2009 Apr 13;206(4):735-42. doi: 10.1084/jem.20081773. Epub 2009 Mar 23. J Exp Med. 2009. PMID: 19307326 Free PMC article.
-
The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease.Cell Metab. 2023 Nov 7;35(11):1852-1871. doi: 10.1016/j.cmet.2023.10.009. Cell Metab. 2023. PMID: 37939656 Free PMC article. Review.
References
-
- Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, et al. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J Immunol. 1992;149:1556–1561. - PubMed
-
- Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. - PubMed
-
- Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu Rev Immunol. 2002;20:29–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

