Down-regulation of cardiac lineage protein (CLP-1) expression in CLP-1 +/- mice affords
- PMID: 18624753
- PMCID: PMC4940775
- DOI: 10.1111/j.1582-4934.2008.00404.x
Down-regulation of cardiac lineage protein (CLP-1) expression in CLP-1 +/- mice affords
Abstract
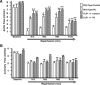
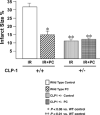
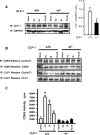
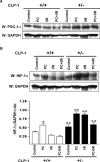
In order to understand the transcriptional mechanism that underlies cell protection to stress, we evaluated the role of CLP-1, a known inhibitor of the transcription elongation complex (pTEFb), in CLP-1 +/- mice hearts. Using the isolated heart model, we observed that the CLP-1 +/- hearts, when subjected to ischaemic stress and evaluated by haemodynamic measurements, exhibit significant cardioprotection. CLP-1 remains associated with the pTEFb complex in the heterozygous hearts, where as it is released in the wild-type hearts suggesting the involvement of pTEFb regulation in cell protection. There was a decrease in Cdk7 and Cdk9 kinase activity and consequently in phosphorylation of serine-5 and serine-2 of Pol II CTD in CLP-1 +/- hearts. However, the levels of mitochondrial proteins, PGC-1alpha and HIF-1alpha, which enhance mitochondrial activity and are implicated in cell survival, were increased in CLP-1 +/- hearts subjected to ischaemic stress compared to that in wild-type CLP-1 +/- hearts treated identically. There was also an increase in the expression of pyruvate dehydrogenase kinase (PDK-1), which facilitates cell adaptation to hypoxic stress. Taken together, our data suggest that regulation of the CLP-1 levels is critical to cellular adaptation of the survival program that protects cardiomyocytes against stress due collectively to a decrease in RNA Pol II phosphorylation but an increase in expression of target proteins that regulate mitochondrial function and metabolic adaptation to stress.
Figures





Similar articles
-
Ablation of the CLP-1 gene leads to down-regulation of the HAND1 gene and abnormality of the left ventricle of the heart and fetal death.Mech Dev. 2004 Jun;121(6):559-72. doi: 10.1016/j.mod.2004.04.012. Mech Dev. 2004. PMID: 15172687
-
Pivotal role of cardiac lineage protein-1 (CLP-1) in transcriptional elongation factor P-TEFb complex formation in cardiac hypertrophy.Cardiovasc Res. 2007 Jul 1;75(1):129-38. doi: 10.1016/j.cardiores.2007.03.019. Epub 2007 Mar 28. Cardiovasc Res. 2007. PMID: 17459355 Free PMC article.
-
Downregulation of cardiac lineage protein-1 confers cardioprotection through the upregulation of redox effectors.FEBS Lett. 2010 Jan 4;584(1):187-93. doi: 10.1016/j.febslet.2009.11.054. FEBS Lett. 2010. PMID: 19931534
-
Cardioprotection by acetylcholine: a novel mechanism via mitochondrial biogenesis and function involving the PGC-1α pathway.J Cell Physiol. 2013 Jun;228(6):1238-48. doi: 10.1002/jcp.24277. J Cell Physiol. 2013. PMID: 23139024
-
Control of cardiac pyruvate dehydrogenase activity in peroxisome proliferator-activated receptor-alpha transgenic mice.Am J Physiol Heart Circ Physiol. 2003 Jul;285(1):H270-6. doi: 10.1152/ajpheart.00852.2002. Epub 2003 Mar 27. Am J Physiol Heart Circ Physiol. 2003. PMID: 12663261
Cited by
-
Small interfering RNA targeting PGC-1α inhibits VEGF expression and tube formation in human retinal vascular endothelial cells.Int J Ophthalmol. 2015 Oct 18;8(5):877-83. doi: 10.3980/j.issn.2222-3959.2015.05.05. eCollection 2015. Int J Ophthalmol. 2015. PMID: 26558195 Free PMC article.
-
Functional Interaction between HEXIM and Hedgehog Signaling during Drosophila Wing Development.PLoS One. 2016 May 13;11(5):e0155438. doi: 10.1371/journal.pone.0155438. eCollection 2016. PLoS One. 2016. PMID: 27176767 Free PMC article.
-
Cross-talk between calcineurin/NFAT and Jak/STAT signalling induces cardioprotective alphaB-crystallin gene expression in response to hypertrophic stimuli.J Cell Mol Med. 2010 Jun;14(6B):1707-16. doi: 10.1111/j.1582-4934.2009.00804.x. Epub 2009 Jun 16. J Cell Mol Med. 2010. PMID: 19538478 Free PMC article.
-
Cardiomyocyte-specific overexpression of HEXIM1 prevents right ventricular hypertrophy in hypoxia-induced pulmonary hypertension in mice.PLoS One. 2012;7(12):e52522. doi: 10.1371/journal.pone.0052522. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300697 Free PMC article.
References
-
- Ananthakrishnan R, Hallam K, Li Q, et al . JAK‐STAT pathway in cardiac ischaemic stress. Vascul Pharmacol. 2005; 43: 353–6. - PubMed
-
- Willis MS, Patterson C. Into the heart: the emerging role of the ubiquitin‐ proteasome system. J Mol Cell Cardiol. 2006; 41: 567–9. - PubMed
-
- Hall G, Hasday JD, Rogers TB. Regulating the regulator: NF‐kappaB signaling in heart. J Mol Cell Cardiol. 2006; 41: 580–91. - PubMed
-
- Das DK, Maulik N. Conversion of death signal into survival signal by redox signaling. Biochemistry. 2004; 69: 10–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

