Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo
- PMID: 18599511
- PMCID: PMC2708103
- DOI: 10.1242/dev.016329
Role for PADI6 and the cytoplasmic lattices in ribosomal storage in oocytes and translational control in the early mouse embryo
Abstract
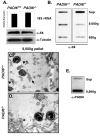
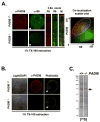
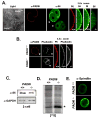
The mechanisms that mediate the establishment of totipotency during the egg-to-embryo transition in mammals remain poorly understood. However, it is clear that unique factors stored in the oocyte cytoplasm are crucial for orchestrating this complex cellular transition. The oocyte cytoplasmic lattices (CPLs) have long been predicted to function as a storage form for the maternal contribution of ribosomes to the early embryo. We recently demonstrated that the CPLs cannot be visualized in Padi6-/- oocytes and that Padi6-/- embryos arrest at the two-cell stage. Here, we present evidence further supporting the association of ribosomes with the CPLs by demonstrating that the sedimentation properties of the small ribosomal subunit protein, S6, are dramatically altered in Padi6-/- oocytes. We also show that the abundance and localization of ribosomal components is dramatically affected in Padi6-/- two-cell embryos and that de novo protein synthesis is also dysregulated in these embryos. Finally, we demonstrate that embryonic genome activation (EGA) is defective in Padi6-/- two-cell embryos. These results suggest that, in mammals, ribosomal components are stored in the oocyte CPLs and are required for protein translation during early development.
Figures


Similar articles
-
Role for PADI6 in securing the mRNA-MSY2 complex to the oocyte cytoplasmic lattices.Cell Cycle. 2017 Feb 16;16(4):360-366. doi: 10.1080/15384101.2016.1261225. Epub 2016 Dec 8. Cell Cycle. 2017. PMID: 27929740 Free PMC article.
-
Potential role for MATER in cytoplasmic lattice formation in murine oocytes.PLoS One. 2010 Sep 7;5(9):e12587. doi: 10.1371/journal.pone.0012587. PLoS One. 2010. PMID: 20830304 Free PMC article.
-
Subcellular localization of cytoplasmic lattice-associated proteins is dependent upon fixation and processing procedures.PLoS One. 2011 Feb 16;6(2):e17226. doi: 10.1371/journal.pone.0017226. PLoS One. 2011. PMID: 21359190 Free PMC article.
-
PADI6: What we know about the elusive fifth member of the peptidyl arginine deiminase family.Philos Trans R Soc Lond B Biol Sci. 2023 Nov 20;378(1890):20220242. doi: 10.1098/rstb.2022.0242. Epub 2023 Oct 2. Philos Trans R Soc Lond B Biol Sci. 2023. PMID: 37778376 Free PMC article. Review.
-
New insights into oocyte cytoplasmic lattice-associated proteins.Trends Genet. 2024 Oct;40(10):880-890. doi: 10.1016/j.tig.2024.06.002. Epub 2024 Jul 1. Trends Genet. 2024. PMID: 38955588 Review.
Cited by
-
The Landscape of Point Mutations in Human Protein Coding Genes Leading to Pregnancy Loss.Int J Mol Sci. 2023 Dec 17;24(24):17572. doi: 10.3390/ijms242417572. Int J Mol Sci. 2023. PMID: 38139401 Free PMC article. Review.
-
Reproductive Outcomes from Maternal Loss of Nlrp2 Are Not Improved by IVF or Embryo Transfer Consistent with Oocyte-Specific Defect.Reprod Sci. 2021 Jul;28(7):1850-1865. doi: 10.1007/s43032-020-00360-x. Epub 2020 Oct 22. Reprod Sci. 2021. PMID: 33090377 Free PMC article.
-
An Overview of the Intrinsic Role of Citrullination in Autoimmune Disorders.J Immunol Res. 2019 Nov 25;2019:7592851. doi: 10.1155/2019/7592851. eCollection 2019. J Immunol Res. 2019. PMID: 31886309 Free PMC article. Review.
-
Novel Homozygous PADI6 Variants in Infertile Females with Early Embryonic Arrest.Front Cell Dev Biol. 2022 Apr 1;10:819667. doi: 10.3389/fcell.2022.819667. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35433708 Free PMC article.
-
Molecular and ultrastuctural changes of rat pre-implantation embryos during two-cell developmental arrest.J Assist Reprod Genet. 2014 Jun;31(6):767-80. doi: 10.1007/s10815-014-0213-4. J Assist Reprod Genet. 2014. PMID: 24658924 Free PMC article.
References
-
-
D. E.H. (Academic Press, New York City, 1986).
-
-
- Schultz RM. Hum Reprod Update. 2002 Jul-Aug;8:323. - PubMed
-
- Bachvarova R, De Leon V. Dev Biol. 1977 Jul 15;58:248. - PubMed
-
- Garcia RB, Pereyra-Alfonso S, Sotelo JR. Differentiation. 1979;14:101. - PubMed
-
- Wassarman PM, Josefowicz WJ. J Morphol. 1978 May;156:209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

