Arf family GTP loading is activated by, and generates, positive membrane curvature
- PMID: 18597672
- PMCID: PMC2518064
- DOI: 10.1042/BJ20081237
Arf family GTP loading is activated by, and generates, positive membrane curvature
Abstract
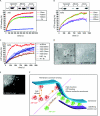
Small G-proteins belonging to the Arf (ADP-ribosylation factor) family serve as regulatory proteins for numerous cellular processes through GTP-dependent recruitment of effector molecules. In the present study we demonstrate that proteins in this family regulate, and are regulated by, membrane curvature. Arf1 and Arf6 were shown to load GTP in a membrane-curvature-dependent manner and stabilize, or further facilitate, changes in membrane curvature through the insertion of an amphipathic helix.
Figures

Comment in
-
Arfs and membrane lipids: sensing, generating and responding to membrane curvature.Biochem J. 2008 Sep 1;414(2):e1-2. doi: 10.1042/BJ20081438. Biochem J. 2008. PMID: 18687059
Similar articles
-
Arfs and membrane lipids: sensing, generating and responding to membrane curvature.Biochem J. 2008 Sep 1;414(2):e1-2. doi: 10.1042/BJ20081438. Biochem J. 2008. PMID: 18687059
-
EFA6 controls Arf1 and Arf6 activation through a negative feedback loop.Proc Natl Acad Sci U S A. 2014 Aug 26;111(34):12378-83. doi: 10.1073/pnas.1409832111. Epub 2014 Aug 11. Proc Natl Acad Sci U S A. 2014. PMID: 25114232 Free PMC article.
-
The structural basis of Arfaptin-mediated cross-talk between Rac and Arf signalling pathways.Nature. 2001 May 10;411(6834):215-9. doi: 10.1038/35075620. Nature. 2001. PMID: 11346801
-
Membrane curvature and the control of GTP hydrolysis in Arf1 during COPI vesicle formation.Biochem Soc Trans. 2005 Aug;33(Pt 4):619-22. doi: 10.1042/BST0330619. Biochem Soc Trans. 2005. PMID: 16042557 Review.
-
Localization and function of Arf family GTPases.Biochem Soc Trans. 2005 Aug;33(Pt 4):639-42. doi: 10.1042/BST0330639. Biochem Soc Trans. 2005. PMID: 16042562 Review.
Cited by
-
The late stage of COPI vesicle fission requires shorter forms of phosphatidic acid and diacylglycerol.Nat Commun. 2019 Jul 30;10(1):3409. doi: 10.1038/s41467-019-11324-4. Nat Commun. 2019. PMID: 31363100 Free PMC article.
-
Biophysical mechanism for ras-nanocluster formation and signaling in plasma membrane.PLoS One. 2009 Jul 9;4(7):e6148. doi: 10.1371/journal.pone.0006148. PLoS One. 2009. PMID: 19587789 Free PMC article.
-
Structural basis for membrane binding and remodeling by the exomer secretory vesicle cargo adaptor.Dev Cell. 2014 Sep 8;30(5):610-24. doi: 10.1016/j.devcel.2014.07.014. Dev Cell. 2014. PMID: 25203211 Free PMC article.
-
Membrane Curvature Sensing by Amphipathic Helices Is Modulated by the Surrounding Protein Backbone.PLoS One. 2015 Sep 14;10(9):e0137965. doi: 10.1371/journal.pone.0137965. eCollection 2015. PLoS One. 2015. PMID: 26366573 Free PMC article.
-
Structural elucidation of how ARF small GTPases induce membrane tubulation for vesicle fission.bioRxiv [Preprint]. 2023 Dec 20:2023.12.19.572083. doi: 10.1101/2023.12.19.572083. bioRxiv. 2023. PMID: 38187566 Free PMC article. Preprint.
References
-
- Gillingham A. K., Munro S. The small G proteins of the Arf family and their regulators. Annu. Rev. Cell Dev. Biol. 2007;23:579–611. - PubMed
-
- D'souza-Schorey C., Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 2006;7:347–358. - PubMed
-
- Amor J. C., Harrison D. H., Kahn R. A., Ringe D. Structure of the human ADP-ribosylation factor 1 complexed with GDP. Nature. 1994;372:704–708. - PubMed
-
- Menetrey J., Macia E., Pasqualato S., Franco M., Cherfils J. Structure of Arf6–GDP suggests a basis for guanine nucleotide exchange factors specificity. Nat. Struct. Biol. 2000;7:466–469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

