Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein
- PMID: 18596047
- PMCID: PMC2882233
- DOI: 10.1074/jbc.M802140200
Palmitoylation and membrane interactions of the neuroprotective chaperone cysteine-string protein
Abstract
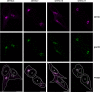
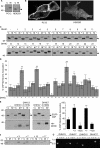
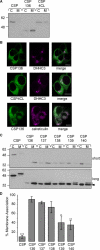
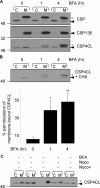
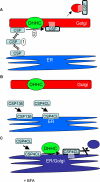
Cysteine-string protein (CSP) is an extensively palmitoylated DnaJ-family chaperone, which exerts an important neuroprotective function. Palmitoylation is required for the intracellular sorting and function of CSP, and thus it is important to understand how this essential modification of CSP is regulated. Recent work identified 23 putative palmitoyl transferases containing a conserved DHHC domain in mammalian cells, and here we show that palmitoylation of CSP is enhanced specifically by co-expression of the Golgi-localized palmitoyl transferases DHHC3, DHHC7, DHHC15, or DHHC17. Indeed, these DHHC proteins promote stable membrane attachment of CSP, which is otherwise cytosolic. An inverse correlation was identified between membrane affinity of unpalmitoylated CSP mutants and subsequent palmitoylation: mutants with an increased membrane affinity localize to the endoplasmic reticulum (ER) and are physically separated from the Golgi-localized DHHC proteins. Palmitoylation of an ER-localized mutant could be rescued by brefeldin A treatment, which promotes the mixing of ER and Golgi membranes. Interestingly though, the palmitoylated mutant remained at the ER following brefeldin A washout and did not traffic to more distal membrane compartments. We propose that CSP has a weak membrane affinity that allows the protein to locate its partner Golgi-localized DHHC proteins directly by membrane "sampling." Mutations that enhance membrane association prevent sampling and lead to accumulation of CSP on cellular membranes such as the ER. The coupling of CSP palmitoylation to Golgi membranes may thus be an important requirement for subsequent sorting.
Figures




Similar articles
-
Endoplasmic reticulum localization of DHHC palmitoyltransferases mediated by lysine-based sorting signals.J Biol Chem. 2011 Nov 11;286(45):39573-84. doi: 10.1074/jbc.M111.272369. Epub 2011 Sep 18. J Biol Chem. 2011. PMID: 21926431 Free PMC article.
-
Dual role of the cysteine-string domain in membrane binding and palmitoylation-dependent sorting of the molecular chaperone cysteine-string protein.Mol Biol Cell. 2006 Nov;17(11):4748-59. doi: 10.1091/mbc.e06-03-0183. Epub 2006 Aug 30. Mol Biol Cell. 2006. PMID: 16943324 Free PMC article.
-
Palmitoylation of the SNAP25 protein family: specificity and regulation by DHHC palmitoyl transferases.J Biol Chem. 2010 Aug 6;285(32):24629-38. doi: 10.1074/jbc.M110.119289. Epub 2010 Jun 2. J Biol Chem. 2010. PMID: 20519516 Free PMC article.
-
Cysteine string protein (CSP) and its role in preventing neurodegeneration.Semin Cell Dev Biol. 2015 Apr;40:153-9. doi: 10.1016/j.semcdb.2015.03.008. Epub 2015 Mar 21. Semin Cell Dev Biol. 2015. PMID: 25800794 Free PMC article. Review.
-
The intracellular dynamic of protein palmitoylation.J Cell Biol. 2010 Dec 27;191(7):1229-38. doi: 10.1083/jcb.201008160. J Cell Biol. 2010. PMID: 21187327 Free PMC article. Review.
Cited by
-
The Thr715Pro variant impairs terminal glycosylation of P-selectin.Thromb Haemost. 2012 Nov;108(5):963-72. doi: 10.1160/TH12-01-0047. Epub 2012 Sep 26. Thromb Haemost. 2012. PMID: 23014585 Free PMC article.
-
The yeast kinase Yck2 has a tripartite palmitoylation signal.Mol Biol Cell. 2011 Aug 1;22(15):2702-15. doi: 10.1091/mbc.E11-02-0115. Epub 2011 Jun 8. Mol Biol Cell. 2011. PMID: 21653825 Free PMC article.
-
Identification of palmitoyltransferase and thioesterase enzymes that control the subcellular localization of axon survival factor nicotinamide mononucleotide adenylyltransferase 2 (NMNAT2).J Biol Chem. 2014 Nov 21;289(47):32858-70. doi: 10.1074/jbc.M114.582338. Epub 2014 Sep 30. J Biol Chem. 2014. PMID: 25271157 Free PMC article.
-
Regulatory effects of protein S-acylation on insulin secretion and insulin action.Open Biol. 2021 Mar;11(3):210017. doi: 10.1098/rsob.210017. Epub 2021 Mar 31. Open Biol. 2021. PMID: 33784857 Free PMC article. Review.
-
Abnormal triaging of misfolded proteins by adult neuronal ceroid lipofuscinosis-associated DNAJC5/CSPα mutants causes lipofuscin accumulation.Autophagy. 2023 Jan;19(1):204-223. doi: 10.1080/15548627.2022.2065618. Epub 2022 May 4. Autophagy. 2023. PMID: 35506243 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

