Do multiple ionic interactions contribute to skeletal muscle fatigue?
- PMID: 18591187
- PMCID: PMC2652190
- DOI: 10.1113/jphysiol.2008.155424
Do multiple ionic interactions contribute to skeletal muscle fatigue?
Abstract
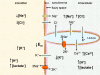
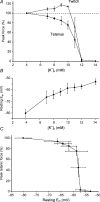
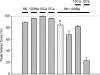
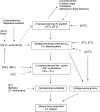
During intense exercise or electrical stimulation of skeletal muscle the concentrations of several ions change simultaneously in interstitial, transverse tubular and intracellular compartments. Consequently the functional effects of multiple ionic changes need to be considered together. A diminished transsarcolemmal K(+) gradient per se can reduce maximal force in non-fatigued muscle suggesting that K(+) causes fatigue. However, this effect requires extremely large, although physiological, K(+) shifts. In contrast, moderate elevations of extracellular [K(+)] ([K(+)](o)) potentiate submaximal contractions, enhance local blood flow and influence afferent feedback to assist exercise performance. Changed transsarcolemmal Na(+), Ca(2+), Cl(-) and H(+) gradients are insufficient by themselves to cause much fatigue but each ion can interact with K(+) effects. Lowered Na(+), Ca(2+) and Cl(-) gradients further impair force by modulating the peak tetanic force-[K(+)](o) and peak tetanic force-resting membrane potential relationships. In contrast, raised [Ca(2+)](o), acidosis and reduced Cl(-) conductance during late fatigue provide resistance against K(+)-induced force depression. The detrimental effects of K(+) are exacerbated by metabolic changes such as lowered [ATP](i), depleted carbohydrate, and possibly reactive oxygen species. We hypothesize that during high-intensity exercise a rundown of the transsarcolemmal K(+) gradient is the dominant cellular process around which interactions with other ions and metabolites occur, thereby contributing to fatigue.
Figures
Similar articles
-
The potassium-glycogen interaction on force and excitability in mouse skeletal muscle: implications for fatigue.J Physiol. 2023 Dec;601(24):5669-5687. doi: 10.1113/JP285129. Epub 2023 Nov 7. J Physiol. 2023. PMID: 37934587
-
Resting membrane potential and intracellular [Na+] at rest, during fatigue and during recovery in rat soleus muscle fibres in situ.J Physiol. 2024 Jul;602(14):3469-3487. doi: 10.1113/JP285870. Epub 2024 Jun 15. J Physiol. 2024. PMID: 38877870
-
Exercise and fatigue: integrating the role of K+, Na+ and Cl- in the regulation of sarcolemmal excitability of skeletal muscle.Eur J Appl Physiol. 2023 Nov;123(11):2345-2378. doi: 10.1007/s00421-023-05270-9. Epub 2023 Aug 16. Eur J Appl Physiol. 2023. PMID: 37584745 Free PMC article. Review.
-
Protective role of extracellular chloride in fatigue of isolated mammalian skeletal muscle.Am J Physiol Cell Physiol. 2004 Sep;287(3):C762-70. doi: 10.1152/ajpcell.00589.2003. Epub 2004 May 19. Am J Physiol Cell Physiol. 2004. PMID: 15151907
-
Muscle K+, Na+, and Cl disturbances and Na+-K+ pump inactivation: implications for fatigue.J Appl Physiol (1985). 2008 Jan;104(1):288-95. doi: 10.1152/japplphysiol.01037.2007. Epub 2007 Oct 25. J Appl Physiol (1985). 2008. PMID: 17962569 Review.
Cited by
-
Quercetin ingestion modifies human motor unit firing patterns and muscle contractile properties.Exp Brain Res. 2021 May;239(5):1567-1579. doi: 10.1007/s00221-021-06085-w. Epub 2021 Mar 19. Exp Brain Res. 2021. PMID: 33742251 Free PMC article.
-
Post-exercise Supplementation of Sodium Bicarbonate Improves Acid Base Balance Recovery and Subsequent High-Intensity Boxing Specific Performance.Front Nutr. 2019 Oct 1;6:155. doi: 10.3389/fnut.2019.00155. eCollection 2019. Front Nutr. 2019. PMID: 31632978 Free PMC article.
-
Unaccustomed eccentric contractions impair plasma K+ regulation in the absence of changes in muscle Na+,K+-ATPase content.PLoS One. 2014 Jun 24;9(6):e101039. doi: 10.1371/journal.pone.0101039. eCollection 2014. PLoS One. 2014. PMID: 24959836 Free PMC article.
-
Effects of membrane depolarization and changes in extracellular [K(+)] on the Ca (2+) transients of fast skeletal muscle fibers. Implications for muscle fatigue.J Muscle Res Cell Motil. 2010 Jul;31(1):13-33. doi: 10.1007/s10974-009-9195-8. Epub 2010 Jan 5. J Muscle Res Cell Motil. 2010. PMID: 20049631 Free PMC article.
-
Chronic Ingestion of Sodium and Potassium Bicarbonate, with Potassium, Magnesium and Calcium Citrate Improves Anaerobic Performance in Elite Soccer Players.Nutrients. 2018 Nov 1;10(11):1610. doi: 10.3390/nu10111610. Nutrients. 2018. PMID: 30388775 Free PMC article.
References
-
- Ahlborg B, Bergström J, Ekelund LG, Hultman E. Muscle glycogen and muscle electrolytes during prolonged physical exercise. Acta Physiol Scand. 1967;70:129–142.
-
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. - PubMed
-
- Atrakchi A, Gray SD, Carlsen RC. Development of soleus muscles in SHR: relationship of muscle deficits to rise in blood pressure. Am J Physiol Cell Physiol. 1994;267:C827–C835. - PubMed
-
- Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in vitro maximal Na+-K+-ATPase activity in well-trained athletes. J Appl Physiol. 2005;98:186–192. - PubMed
-
- Balog EM, Fitts RH. Effects of depolarization and low intracellular pH on charge movement currents of frog skeletal muscle fibers. J Appl Physiol. 2001;90:228–234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

