An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells
- PMID: 18583533
- PMCID: PMC2492636
- DOI: 10.1104/pp.108.120238
An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells
Erratum in
- Plant Physiol. 2008 Oct;148(2):1182. David Ho, Thun-Hua [corrected to Ho, Tuan-Hua]
Abstract
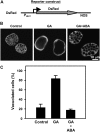
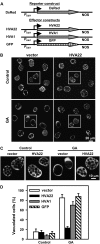
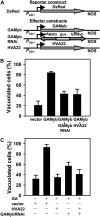
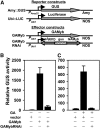
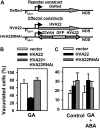
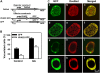
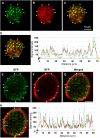
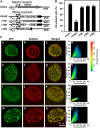
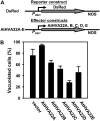
Plant HVA22 is a unique abscisic acid (ABA)/stress-induced protein first isolated from barley (Hordeum vulgare) aleurone cells. Its yeast homolog, Yop1p, functions in vesicular trafficking and in the endoplasmic reticulum (ER) network in vivo. To examine the roles of plant HVA22, barley HVA22 was ectopically expressed in barley aleurone cells. Overexpression of HVA22 proteins inhibited gibberellin (GA)-induced formation of large digestive vacuoles, which is an important aspect of GA-induced programmed cell death in aleurone cells. The effect of HVA22 was specific, because overexpression of green fluorescent protein or another ABA-induced protein, HVA1, did not lead to the same effect. HVA22 acts downstream of the transcription factor GAMyb, which activates programmed cell death and other GA-mediated processes. Moreover, expression of HVA22:green fluorescent protein fusion proteins showed network and punctate fluorescence patterns, which were colocalized with an ER marker, BiP:RFP, and a Golgi marker, ST:mRFP, respectively. In particular, the transmembrane domain 2 was critical for protein localization and stability. Ectopic expression of the most phylogenetically similar Arabidopsis (Arabidopsis thaliana) homolog, AtHVA22D, also resulted in the inhibition of vacuolation to a similar level as HVA22, indicating function conservation between barley HVA22 and some Arabidopsis homologs. Taken together, we show that HVA22 is an ER- and Golgi-localized protein capable of negatively regulating GA-mediated vacuolation/programmed cell death in barley aleurone cells. We propose that ABA induces the accumulation of HVA22 proteins to inhibit vesicular trafficking involved in nutrient mobilization to delay coalescence of protein storage vacuoles as part of its role in regulating seed germination and seedling growth.
Figures
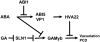
Similar articles
-
The stress- and abscisic acid-induced barley gene HVA22: developmental regulation and homologues in diverse organisms.Plant Mol Biol. 2001 Feb;45(3):327-40. doi: 10.1023/a:1006460231978. Plant Mol Biol. 2001. PMID: 11292078
-
cPrG-HCl a potential H+/Cl- symporter prevents acidification of storage vacuoles in aleurone cells and inhibits GA-dependent hydrolysis of storage protein and phytate.Plant J. 2003 Jul;35(2):154-63. doi: 10.1046/j.1365-313x.2003.01789.x. Plant J. 2003. PMID: 12848822
-
Gibberellin signaling in barley aleurone cells. Control of SLN1 and GAMYB expression.Plant Physiol. 2002 May;129(1):191-200. doi: 10.1104/pp.010918. Plant Physiol. 2002. PMID: 12011350 Free PMC article.
-
Proteomes of the barley aleurone layer: A model system for plant signalling and protein secretion.Proteomics. 2011 May;11(9):1595-605. doi: 10.1002/pmic.201000656. Epub 2011 Mar 23. Proteomics. 2011. PMID: 21433287 Review.
-
Programmed cell death in cereal aleurone.Plant Mol Biol. 2000 Oct;44(3):255-66. doi: 10.1023/a:1026584207243. Plant Mol Biol. 2000. PMID: 11199387 Review.
Cited by
-
Identification of small RNAs during cold acclimation in Arabidopsis thaliana.BMC Plant Biol. 2020 Jun 29;20(1):298. doi: 10.1186/s12870-020-02511-3. BMC Plant Biol. 2020. PMID: 32600430 Free PMC article.
-
Differentiation mechanism and function of the cereal aleurone cells and hormone effects on them.Plant Cell Rep. 2014 Nov;33(11):1779-87. doi: 10.1007/s00299-014-1654-z. Epub 2014 Jul 10. Plant Cell Rep. 2014. PMID: 25007781 Review.
-
De novo transcriptome in roots of switchgrass (Panicum virgatum L.) reveals gene expression dynamic and act network under alkaline salt stress.BMC Genomics. 2021 Jan 28;22(1):82. doi: 10.1186/s12864-021-07368-w. BMC Genomics. 2021. PMID: 33509088 Free PMC article.
-
The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants.Sci Rep. 2021 Jan 11;11(1):354. doi: 10.1038/s41598-020-79770-5. Sci Rep. 2021. PMID: 33432010 Free PMC article.
-
Heat-induced proteomic changes in anthers of contrasting rice genotypes under variable stress regimes.Front Plant Sci. 2023 Jan 13;13:1083971. doi: 10.3389/fpls.2022.1083971. eCollection 2022. Front Plant Sci. 2023. PMID: 36756226 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

