Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/- regulatory T cells in allergic asthma
- PMID: 18581330
- PMCID: PMC4217522
- DOI: 10.1002/eji.200738082
Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/- regulatory T cells in allergic asthma
Abstract
CD4+CD25(hi)CD127(lo/-) regulatory T cells (Treg) have been implicated in the resolution of asthma-associated inflammation while the opposite role of CD4+ invariant NKT (iNKT) cells has been the subject of recent investigations. Studies here focused on mechanisms of interaction between CD4+ iNKT cells and Treg to further explore their roles in allergic asthma (AA). Flow cytometry analysis revealed a significant increase in the expression of the natural cytotoxicity receptors NKp30 and NKp46 by CD4+ iNKT cells in AA subjects compared to healthy controls (HC) and non-allergic asthmatics (NA). Subsequent intracellular staining showed that CD4+ iNKT cells also expressed higher levels of granzyme B and perforin in AA than HC. In in vitro killing assays, AA CD4+ iNKT cells selectively killed autologous Treg, but not CD4+CD25- T cells, more potently than HC and NA counterparts. This increased cytotoxicity positively correlated with asthma severity and granzyme B/perforin expression of CD4+ iNKT cells. Furthermore, it could be abrogated by either inhibition of the granzyme B-/perforin-dependent cell death pathway or oral corticosteroid administration. Altogether, these findings suggest that increased cytotoxicity of CD4+ iNKT cells against Treg might contribute to dysfunctional cellular interactions in AA.
Conflict of interest statement
Figures




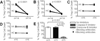
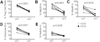
Similar articles
-
Reciprocal granzyme/perforin-mediated death of human regulatory and responder T cells is regulated by interleukin-2 (IL-2).J Mol Med (Berl). 2010 Jun;88(6):577-88. doi: 10.1007/s00109-010-0602-9. Epub 2010 Mar 12. J Mol Med (Berl). 2010. PMID: 20225066 Free PMC article.
-
Altered phosphorylated signal transducer and activator of transcription profile of CD4+CD161+ T cells in asthma: modulation by allergic status and oral corticosteroids.J Allergy Clin Immunol. 2007 Dec;120(6):1441-8. doi: 10.1016/j.jaci.2007.08.012. Epub 2007 Oct 24. J Allergy Clin Immunol. 2007. PMID: 17919711 Free PMC article.
-
Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma.J Allergy Clin Immunol. 2007 May;119(5):1258-66. doi: 10.1016/j.jaci.2007.02.023. Epub 2007 Apr 6. J Allergy Clin Immunol. 2007. PMID: 17412402
-
Invariant natural killer T (iNKT) cells in asthma: a novel insight into the pathogenesis of asthma and the therapeutic implication of glycolipid ligands for allergic diseases.Allergol Int. 2007 Mar;56(1):7-14. doi: 10.2332/allergolint.R-06-137. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259804 Review.
-
iNKT cells in allergic disease.Curr Top Microbiol Immunol. 2007;314:269-91. doi: 10.1007/978-3-540-69511-0_11. Curr Top Microbiol Immunol. 2007. PMID: 17593665 Review.
Cited by
-
Revisiting regulatory T cells as modulators of innate immune response and inflammatory diseases.Front Immunol. 2023 Oct 20;14:1287465. doi: 10.3389/fimmu.2023.1287465. eCollection 2023. Front Immunol. 2023. PMID: 37928540 Free PMC article. Review.
-
Natural killer T cells in allergic asthma: implications for the development of novel immunotherapeutical strategies.Front Immunol. 2024 Apr 2;15:1364774. doi: 10.3389/fimmu.2024.1364774. eCollection 2024. Front Immunol. 2024. PMID: 38629075 Free PMC article. Review.
-
Therapeutic potential of FOXP3(+) regulatory T cells and their interactions with dendritic cells.Hum Immunol. 2009 May;70(5):294-9. doi: 10.1016/j.humimm.2009.02.007. Epub 2009 Feb 21. Hum Immunol. 2009. PMID: 19236900 Free PMC article. Review.
-
Regulatory T cells regulate the distribution of natural killer T cells through CD39 signal transduction in asthma.Hum Cell. 2019 Apr;32(2):141-149. doi: 10.1007/s13577-018-00226-0. Epub 2018 Dec 11. Hum Cell. 2019. PMID: 30539423
-
STAT3 governs hyporesponsiveness and granzyme B-dependent suppressive capacity in human CD4+ T cells.FASEB J. 2015 Mar;29(3):759-71. doi: 10.1096/fj.14-257584. Epub 2014 Nov 14. FASEB J. 2015. PMID: 25398767 Free PMC article.
References
-
- Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N. Engl. J. Med. 1991;325:1067–1071. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 1992;326:298–304. - PubMed
-
- Umetsu DT, Dekruyff RH. Immune dysregulation in asthma. Curr. Opin. Immunol. 2006;18:727–732. - PubMed
-
- Robinson DS. The role of regulatory T lymphocytes in asthma pathogenesis. Curr. Allergy Asthma Rep. 2005;5:136–141. - PubMed
-
- Baecher-Allan C, Viglietta V, Hafler DA. Human CD4+CD25+ regulatory T cells. Semin. Immunol. 2004;16:89–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

