Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1
- PMID: 18579743
- PMCID: PMC2739057
- DOI: 10.1523/JNEUROSCI.1701-08.2008
Endocytosis of prion protein is required for ERK1/2 signaling induced by stress-inducible protein 1
Abstract
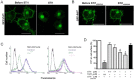
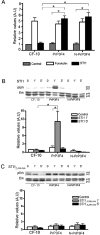
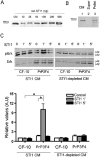
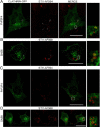
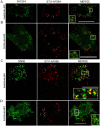
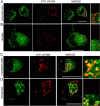
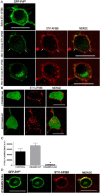
The secreted cochaperone STI1 triggers activation of protein kinase A (PKA) and ERK1/2 signaling by interacting with the cellular prion (PrP(C)) at the cell surface, resulting in neuroprotection and increased neuritogenesis. Here, we investigated whether STI1 triggers PrP(C) trafficking and tested whether this process controls PrP(C)-dependent signaling. We found that STI1, but not a STI1 mutant unable to bind PrP(C), induced PrP(C) endocytosis. STI1-induced signaling did not occur in cells devoid of endogenous PrP(C); however, heterologous expression of PrP(C) reconstituted both PKA and ERK1/2 activation. In contrast, a PrP(C) mutant lacking endocytic activity was unable to promote ERK1/2 activation induced by STI1, whereas it reconstituted PKA activity in the same condition, suggesting a key role of endocytosis in the former process. The activation of ERK1/2 by STI1 was transient and appeared to depend on the interaction of the two proteins at the cell surface or shortly after internalization. Moreover, inhibition of dynamin activity by expression of a dominant-negative mutant caused the accumulation and colocalization of these proteins at the plasma membrane, suggesting that both proteins use a dynamin-dependent internalization pathway. These results show that PrP(C) endocytosis is a necessary step to modulate STI1-dependent ERK1/2 signaling involved in neuritogenesis.
Figures


Similar articles
-
Role of alpha7 nicotinic acetylcholine receptor in calcium signaling induced by prion protein interaction with stress-inducible protein 1.J Biol Chem. 2010 Nov 19;285(47):36542-50. doi: 10.1074/jbc.M110.157263. Epub 2010 Sep 13. J Biol Chem. 2010. PMID: 20837487 Free PMC article.
-
Loss of STI1-mediated neuronal survival and differentiation in disease-associated mutations of prion protein.J Neurochem. 2018 Jun;145(5):409-416. doi: 10.1111/jnc.14305. Epub 2018 Apr 2. J Neurochem. 2018. PMID: 29337365
-
Prion protein and its ligand stress inducible protein 1 regulate astrocyte development.Glia. 2009 Oct;57(13):1439-49. doi: 10.1002/glia.20861. Glia. 2009. PMID: 19243016
-
Cellular prion protein signaling in serotonergic neuronal cells.Ann N Y Acad Sci. 2007 Jan;1096:106-19. doi: 10.1196/annals.1397.076. Ann N Y Acad Sci. 2007. PMID: 17405922 Review.
-
Mechanism of the metal-mediated endocytosis of the prion protein.Biochem Soc Trans. 2008 Dec;36(Pt 6):1272-6. doi: 10.1042/BST0361272. Biochem Soc Trans. 2008. PMID: 19021539 Review.
Cited by
-
The prion protein ligand, stress-inducible phosphoprotein 1, regulates amyloid-β oligomer toxicity.J Neurosci. 2013 Oct 16;33(42):16552-64. doi: 10.1523/JNEUROSCI.3214-13.2013. J Neurosci. 2013. PMID: 24133259 Free PMC article.
-
MEK1 transduces the prion protein N2 fragment antioxidant effects.Cell Mol Life Sci. 2015 Apr;72(8):1613-29. doi: 10.1007/s00018-014-1777-y. Epub 2014 Nov 13. Cell Mol Life Sci. 2015. PMID: 25391659 Free PMC article.
-
Show Me Your Friends and I Tell You Who You Are: The Many Facets of Prion Protein in Stroke.Cells. 2020 Jul 2;9(7):1609. doi: 10.3390/cells9071609. Cells. 2020. PMID: 32630841 Free PMC article. Review.
-
Regulation of Amyloid β Oligomer Binding to Neurons and Neurotoxicity by the Prion Protein-mGluR5 Complex.J Biol Chem. 2016 Oct 14;291(42):21945-21955. doi: 10.1074/jbc.M116.738286. Epub 2016 Aug 25. J Biol Chem. 2016. PMID: 27563063 Free PMC article.
-
Prion protein binding to HOP modulates the migration and invasion of colorectal cancer cells.Clin Exp Metastasis. 2016 Jun;33(5):441-51. doi: 10.1007/s10585-016-9788-8. Epub 2016 Apr 25. Clin Exp Metastasis. 2016. PMID: 27112151
References
-
- Americo TA, Chiarini LB, Linden R. Signaling induced by hop/STI-1 depends on endocytosis. Biochem Biophys Res Commun. 2007;358:620–625. - PubMed
-
- Barbosa J, Jr, Ferreira LT, Martins-Silva C, Santos MS, Torres GE, Caron MG, Gomez MV, Ferguson SS, Prado MA, Prado VF. Trafficking of the vesicular acetylcholine transporter in SN56 cells: a dynamin-sensitive step and interaction with the AP-2 adaptor complex. J Neurochem. 2002;82:1221–1228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
