Mitochondrial dynamics and apoptosis
- PMID: 18559474
- PMCID: PMC2732420
- DOI: 10.1101/gad.1658508
Mitochondrial dynamics and apoptosis
Abstract
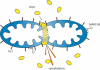
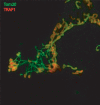
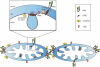
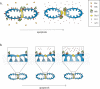
In healthy cells, mitochondria continually divide and fuse to form a dynamic interconnecting network. The molecular machinery that mediates this organelle fission and fusion is necessary to maintain mitochondrial integrity, perhaps by facilitating DNA or protein quality control. This network disintegrates during apoptosis at the time of cytochrome c release and prior to caspase activation, yielding more numerous and smaller mitochondria. Recent work shows that proteins involved in mitochondrial fission and fusion also actively participate in apoptosis induction. This review will cover the recent advances and presents competing models on how the mitochondrial fission and fusion machinery may intersect apoptosis pathways.
Figures

Similar articles
-
[Mitochondrial dynamics regulated by fusion and fission].Tanpakushitsu Kakusan Koso. 2005 Jul;50(8):931-9. Tanpakushitsu Kakusan Koso. 2005. PMID: 16001798 Review. Japanese. No abstract available.
-
Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics.Mol Cell. 2006 Mar 17;21(6):761-73. doi: 10.1016/j.molcel.2006.01.034. Mol Cell. 2006. PMID: 16543146
-
[The comeback of mitochondria in Drosophila apoptosis].Med Sci (Paris). 2016 May;32(5):478-84. doi: 10.1051/medsci/20163205014. Epub 2016 May 25. Med Sci (Paris). 2016. PMID: 27225920 Review. French.
-
Carboxy-Terminal Modulator Protein (CTMP) is a mitochondrial protein that sensitizes cells to apoptosis.Cell Signal. 2009 Apr;21(4):639-50. doi: 10.1016/j.cellsig.2009.01.016. Epub 2009 Jan 8. Cell Signal. 2009. PMID: 19168129
-
Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis.Cell Death Differ. 2007 Jun;14(6):1086-94. doi: 10.1038/sj.cdd.4402107. Epub 2007 Mar 2. Cell Death Differ. 2007. PMID: 17332775
Cited by
-
Inhibition of ER stress attenuates kidney injury and apoptosis induced by 3-MCPD via regulating mitochondrial fission/fusion and Ca2+ homeostasis.Cell Biol Toxicol. 2021 Oct;37(5):795-809. doi: 10.1007/s10565-021-09589-x. Epub 2021 Mar 2. Cell Biol Toxicol. 2021. PMID: 33651226
-
The effect of fasting or calorie restriction on mitophagy induction: a literature review.J Cachexia Sarcopenia Muscle. 2020 Dec;11(6):1447-1458. doi: 10.1002/jcsm.12611. Epub 2020 Aug 27. J Cachexia Sarcopenia Muscle. 2020. PMID: 32856431 Free PMC article. Review.
-
MicroRNA-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity.Cell Death Dis. 2015 Mar 12;6(3):e1677. doi: 10.1038/cddis.2015.41. Cell Death Dis. 2015. PMID: 25766316 Free PMC article.
-
Mitochondria in traumatic brain injury and mitochondrial-targeted multipotential therapeutic strategies.Br J Pharmacol. 2012 Oct;167(4):699-719. doi: 10.1111/j.1476-5381.2012.02025.x. Br J Pharmacol. 2012. PMID: 23003569 Free PMC article. Review.
-
The mycotoxin viriditoxin induces leukemia- and lymphoma-specific apoptosis by targeting mitochondrial metabolism.Cell Death Dis. 2022 Nov 8;13(11):938. doi: 10.1038/s41419-022-05356-w. Cell Death Dis. 2022. PMID: 36347842 Free PMC article.
References
-
- Abdelwahid E., Yokokura T., Krieser R.J., Balasundaram S., Fowle W.H., White K. Mitochondrial disruption in Drosophila apoptosis. Dev. Cell. 2007;12:793–806. - PubMed
-
- Arnoult D., Bartle L.M., Skaletskaya A., Poncet D., Zamzami N., Park P.U., Sharpe J., Youle R.J., Goldmacher V.S. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. 2004;101:7988–7993. - PMC - PubMed
-
- Arnoult D., Grodet A., Lee Y.J., Estaquier J., Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 2005a;280:35742–35750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
