Fate mapping and lineage analyses demonstrate the production of a large number of striatal neuroblasts after transforming growth factor alpha and noggin striatal infusions into the dopamine-depleted striatum
- PMID: 18556510
- PMCID: PMC2649803
- DOI: 10.1634/stemcells.2008-0080
Fate mapping and lineage analyses demonstrate the production of a large number of striatal neuroblasts after transforming growth factor alpha and noggin striatal infusions into the dopamine-depleted striatum
Abstract
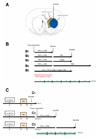
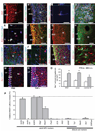
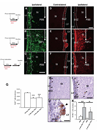
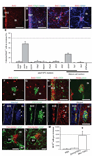
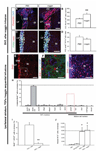
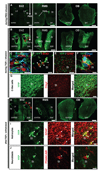
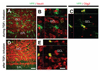
Infusion of transforming growth factor alpha (TGFalpha) into the adult dopamine (DA)-depleted striatum generates a local population of nestin(+)/proliferating cell nuclear antigen (PCNA)(+) newborn cells. The precise origin and fate of these new striatal cells are unknown, making it difficult to direct them for neural repair in Parkinson's disease. Experiments in rats using 5-bromo-2'-deoxyuridine (BrdU) to label neural progenitor cells showed that during TGFalpha infusion in the DA-depleted striatum, newborn striatal cells formed a homogeneous population of precursors, with the majority coexpressing nestin, Mash1, Olig2, and epidermal growth factor receptor, consistent with the phenotype of multipotent C cells. Upon TGFalpha pump withdrawal, the subventricular zone (SVZ) was repopulated by neuroblasts. Strikingly, during this period, numerous clusters of doublecortin(+)/polysialylated neuronal cell adhesion molecule(+) neuroblasts were also produced in the ipsilateral medial striatum. In parallel, striatal BrdU(+)/glial fibrillary acidic protein(+) astrocytes were generated, but no BrdU(+)/O4(+)/CNPase(+) oligodendrocytes were generated. Infusion of the neuralizing bone morphogenetic protein antagonist noggin after TGFalpha pump withdrawal increased the neuroblast-to-astrocyte ratio among new striatal cells by blocking glial differentiation but did not alter striatal neurogenesis. At no time or treatment condition were differentiated neurons generated, including DA neurons. Using 6-hydroxydopamine-lesioned nestin-CreER(T2)/R26R-YFP mice that allow genetic fate-mapping of SVZ nestin(+) cells, we show that TGFalpha-generated striatal cells originate from SVZ nestin(+) precursors that confirmed data from the rats on the phenotype and fate of striatal nestin(+)/PCNA(+) cells upon TGFalpha withdrawal. This work demonstrates that a large population of multipotent striatal C-like cells can be generated in the DA-depleted striatum that do not spontaneously differentiate into DA neurons.
Figures
Similar articles
-
Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice.Glia. 2009 Aug 1;57(10):1115-29. doi: 10.1002/glia.20835. Glia. 2009. PMID: 19115384 Free PMC article.
-
Intrastriatal transforming growth factor alpha delivery to a model of Parkinson's disease induces proliferation and migration of endogenous adult neural progenitor cells without differentiation into dopaminergic neurons.J Neurosci. 2004 Oct 13;24(41):8924-31. doi: 10.1523/JNEUROSCI.2344-04.2004. J Neurosci. 2004. PMID: 15483111 Free PMC article.
-
Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone.Eur J Neurosci. 2002 Dec;16(12):2351-7. doi: 10.1046/j.1460-9568.2002.02412.x. Eur J Neurosci. 2002. PMID: 12492430
-
Nestin-expressing progenitor cells: function, identity and therapeutic implications.Cell Mol Life Sci. 2018 Jun;75(12):2177-2195. doi: 10.1007/s00018-018-2794-z. Epub 2018 Mar 14. Cell Mol Life Sci. 2018. PMID: 29541793 Free PMC article. Review.
-
Distribution, contribution and regulation of nestin+ cells.J Adv Res. 2024 Jul;61:47-63. doi: 10.1016/j.jare.2023.08.013. Epub 2023 Aug 28. J Adv Res. 2024. PMID: 37648021 Free PMC article. Review.
Cited by
-
Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia.Prog Neurobiol. 2011 Jan;93(1):13-24. doi: 10.1016/j.pneurobio.2010.09.004. Epub 2010 Oct 13. Prog Neurobiol. 2011. PMID: 20950668 Free PMC article. Review.
-
An endogenous vitamin K-dependent mechanism regulates cell proliferation in the brain subventricular stem cell niche.Stem Cells. 2012 Apr;30(4):719-31. doi: 10.1002/stem.1045. Stem Cells. 2012. PMID: 22290807 Free PMC article.
-
Neuroblasts contribute to oligodendrocytes generation upon demyelination in the adult mouse brain.iScience. 2022 Sep 13;25(10):105102. doi: 10.1016/j.isci.2022.105102. eCollection 2022 Oct 21. iScience. 2022. PMID: 36185360 Free PMC article.
-
Towards a Better Treatment Option for Parkinson's Disease: A Review of Adult Neurogenesis.Neurochem Res. 2016 Dec;41(12):3161-3170. doi: 10.1007/s11064-016-2053-3. Epub 2016 Sep 10. Neurochem Res. 2016. PMID: 27613619 Review.
-
Using stem cells and iPS cells to discover new treatments for Parkinson's disease.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1(0 1):S14-6. doi: 10.1016/S1353-8020(11)70007-4. Parkinsonism Relat Disord. 2012. PMID: 22166414 Free PMC article. Review.
References
-
- Hoglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. - PubMed
-
- Isacson O. The production and use of cells as therapeutic agents in neurodegenerative diseases. Lancet Neurol. 2003;2:417–424. - PubMed
-
- Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson's disease by stem cells. Ann Neurol. 2003;53 Suppl 3:S135–S146. discussion S146-138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

