Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells
- PMID: 18555660
- PMCID: PMC2771589
- DOI: 10.1016/j.bbi.2008.04.006
Stimulatory and suppressive signal transduction regulates vasoactive intestinal peptide receptor-1 (VPAC-1) in primary mouse CD4 T cells
Abstract
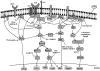
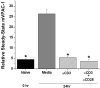
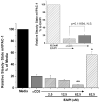
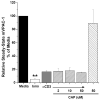
Vasoactive intestinal peptide receptor-1 (VPAC-1) is an anti-proliferative, G-protein coupled receptor that is highly expressed on naïve T cells, and has been reported to be downregulated upon T cell activation. The T cell signaling molecules involved in mediating low VPAC-1 levels have not been identified. Therefore, to gain a greater understanding into this regulation, this study investigated the signaling pathways that regulate (VPAC-1) in murine, primary CD4 T cells. To this end, murine, splenic CD4 T cells were pretreated separately with 10 different pharmacological inhibitors and incubated +/- anti-CD3 for 24h. Total RNA was isolated, and VPAC-1 mRNA levels were measured by qPCR. Our results support that JNK kinases, downstream from the protein kinase, Zap70, are involved in suppressive regulation of VPAC-1 steady-state mRNA levels after anti-CD3 treatment. In contrast, inhibitors against PKC, ERK, p38, Zap70 and Rac1 supported a stimulatory influence in VPAC-1 regulation in the absence of T cell signaling. By studying the signaling pathways that regulate VPAC-1 in T cells, we can gain greater insight into the role of this anti-inflammatory receptor in autoimmunity and infectious diseases.
Figures







Similar articles
-
A transcriptionally permissive epigenetic landscape at the vasoactive intestinal peptide receptor-1 promoter suggests a euchromatin nuclear position in murine CD4 T cells.Regul Pept. 2009 Nov 27;158(1-3):68-76. doi: 10.1016/j.regpep.2009.08.010. Epub 2009 Sep 1. Regul Pept. 2009. PMID: 19729043 Free PMC article.
-
TCR signaling and environment affect vasoactive intestinal peptide receptor-1 (VPAC-1) expression in primary mouse CD4 T cells.Brain Behav Immun. 2008 Oct;22(7):1032-1040. doi: 10.1016/j.bbi.2008.04.005. Epub 2008 Jun 4. Brain Behav Immun. 2008. PMID: 18534815 Free PMC article.
-
Toll-like receptor stimulation differentially regulates vasoactive intestinal peptide type 2 receptor in macrophages.J Cell Mol Med. 2009 Sep;13(9B):3209-17. doi: 10.1111/j.1582-4934.2009.00662.x. J Cell Mol Med. 2009. PMID: 20196778 Free PMC article.
-
Differential expression of vasoactive intestinal polypeptide receptor 1 and 2 mRNA in murine intestinal T lymphocyte subtypes.J Neuroendocrinol. 2001 Sep;13(9):818-25. doi: 10.1046/j.1365-2826.2001.00703.x. J Neuroendocrinol. 2001. PMID: 11578532
-
VPAC and PAC receptors: From ligands to function.Pharmacol Ther. 2009 Mar;121(3):294-316. doi: 10.1016/j.pharmthera.2008.11.006. Epub 2008 Dec 6. Pharmacol Ther. 2009. PMID: 19109992 Review.
Cited by
-
A transcriptionally permissive epigenetic landscape at the vasoactive intestinal peptide receptor-1 promoter suggests a euchromatin nuclear position in murine CD4 T cells.Regul Pept. 2009 Nov 27;158(1-3):68-76. doi: 10.1016/j.regpep.2009.08.010. Epub 2009 Sep 1. Regul Pept. 2009. PMID: 19729043 Free PMC article.
-
Vasoactive intestinal peptide signaling axis in human leukemia.World J Biol Chem. 2011 Jun 26;2(6):146-60. doi: 10.4331/wjbc.v2.i6.146. World J Biol Chem. 2011. PMID: 21765981 Free PMC article.
-
Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents.Brain Behav Immun. 2008 Nov;22(8):1146-51. doi: 10.1016/j.bbi.2008.06.001. Epub 2008 Jun 14. Brain Behav Immun. 2008. PMID: 18598752 Free PMC article. Review.
-
Identification of the early VIP-regulated transcriptome and its associated, interactome in resting and activated murine CD4 T cells.Mol Immunol. 2010 Mar;47(6):1181-94. doi: 10.1016/j.molimm.2010.01.003. Epub 2010 Feb 1. Mol Immunol. 2010. PMID: 20117839 Free PMC article.
-
Characterization and use of a rabbit-anti-mouse VPAC1 antibody by flow cytometry.J Immunol Methods. 2012 Feb 28;376(1-2):20-31. doi: 10.1016/j.jim.2011.10.009. Epub 2011 Nov 4. J Immunol Methods. 2012. PMID: 22079255 Free PMC article.
References
-
- Béné MC. What is ZAP-70? Cytometry B Clin Cytom. 2006 Jul 15;70(4):204–8. - PubMed
-
- Bruno S, Ardelt B, Skierski JS, Traganos F, Darzynkiewicz Z. Different effects of Staurosporine, an inhibitor of protein kinases, on the cell cycle and chromatin structure of normal and leukemic lymphocytes. Cancer Res. 1992;52:470–473. - PubMed
-
- Clements JL, Boerth NJ, Lee JR, Koretzky GA. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu Rev Immunol. 1999;17:89–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

