Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells
- PMID: 18509084
- PMCID: PMC2532803
- DOI: 10.1182/blood-2007-12-128843
Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells
Abstract
Adoptive immunotherapy with T cells expressing a tumor-specific chimeric T-cell receptor is a promising approach to cancer therapy that has not previously been explored for the treatment of lymphoma in human subjects. We report the results of a proof-of-concept clinical trial in which patients with relapsed or refractory indolent B-cell lymphoma or mantle cell lymphoma were treated with autologous T cells genetically modified by electroporation with a vector plasmid encoding a CD20-specific chimeric T-cell receptor and neomycin resistance gene. Transfected cells were immunophenotypically similar to CD8(+) effector cells and showed CD20-specific cytotoxicity in vitro. Seven patients received a total of 20 T-cell infusions, with minimal toxicities. Modified T cells persisted in vivo 1 to 3 weeks in the first 3 patients, who received T cells produced by limiting dilution methods, but persisted 5 to 9 weeks in the next 4 patients who received T cells produced in bulk cultures followed by 14 days of low-dose subcutaneous interleukin-2 (IL-2) injections. Of the 7 treated patients, 2 maintained a previous complete response, 1 achieved a partial response, and 4 had stable disease. These results show the safety, feasibility, and potential antitumor activity of adoptive T-cell therapy using this approach. This trial was registered at www.clinicaltrials.gov as #NCT00012207.
Figures
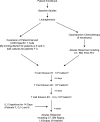
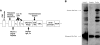


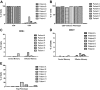


Comment in
-
Test-driving CARs.Blood. 2008 Sep 15;112(6):2172-3. doi: 10.1182/blood-2008-06-163006. Blood. 2008. PMID: 18779394 No abstract available.
Similar articles
-
A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma.Biol Blood Marrow Transplant. 2005 Mar;11(3):181-7. doi: 10.1016/j.bbmt.2004.11.019. Biol Blood Marrow Transplant. 2005. PMID: 15744236 Clinical Trial.
-
Combining a CD20 chimeric antigen receptor and an inducible caspase 9 suicide switch to improve the efficacy and safety of T cell adoptive immunotherapy for lymphoma.PLoS One. 2013 Dec 17;8(12):e82742. doi: 10.1371/journal.pone.0082742. eCollection 2013. PLoS One. 2013. PMID: 24358223 Free PMC article.
-
CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results.Blood. 2012 Apr 26;119(17):3940-50. doi: 10.1182/blood-2011-10-387969. Epub 2012 Feb 3. Blood. 2012. PMID: 22308288 Free PMC article.
-
Experiences with Glofitamab Administration following CAR T Therapy in Patients with Relapsed Mantle Cell Lymphoma.Cells. 2022 Sep 2;11(17):2747. doi: 10.3390/cells11172747. Cells. 2022. PMID: 36078155 Free PMC article. Review.
-
Immunotherapy in mantle cell lymphoma: anti-CD20-based therapy and beyond.Am J Hematol. 2008 Feb;83(2):144-9. doi: 10.1002/ajh.21036. Am J Hematol. 2008. PMID: 17722077 Review.
Cited by
-
Mesothelin-targeted agents in clinical trials and in preclinical development.Mol Cancer Ther. 2012 Mar;11(3):517-25. doi: 10.1158/1535-7163.MCT-11-0454. Epub 2012 Feb 17. Mol Cancer Ther. 2012. PMID: 22351743 Free PMC article. Review.
-
Chimeric antigen receptor-modified T cells for acute lymphoid leukemia.N Engl J Med. 2013 Apr 18;368(16):1509-1518. doi: 10.1056/NEJMoa1215134. Epub 2013 Mar 25. N Engl J Med. 2013. PMID: 23527958 Free PMC article.
-
Pre-clinical efficacy of CD20-targeted chimeric antigen receptor T cells for non-Hodgkin's lymphoma.Discov Oncol. 2022 Nov 9;13(1):122. doi: 10.1007/s12672-022-00588-w. Discov Oncol. 2022. PMID: 36352168 Free PMC article.
-
CD19/CD20 Bispecific Chimeric Antigen Receptor (CAR) in Naive/Memory T Cells for the Treatment of Relapsed or Refractory Non-Hodgkin Lymphoma.Cancer Discov. 2023 Mar 1;13(3):580-597. doi: 10.1158/2159-8290.CD-22-0964. Cancer Discov. 2023. PMID: 36416874 Free PMC article.
-
CD20 CAR T cells safely and reversibly ablate B cell follicles in a non-human primate model of HIV persistence.Mol Ther. 2024 May 1;32(5):1238-1251. doi: 10.1016/j.ymthe.2024.02.030. Epub 2024 Feb 27. Mol Ther. 2024. PMID: 38414244
References
-
- McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. - PubMed
-
- Foran JM, Rohatiner AZ, Cunningham D, et al. European phase II study of rituximab (chimeric anti-CD20 monoclonal antibody) for patients with newly diagnosed mantle-cell lymphoma and previously treated mantle-cell lymphoma, immunocytoma, and small B-cell lymphocytic lymphoma. J Clin Oncol. 2000;18:317–324. - PubMed
-
- Czuczman MS, Grillo-Lopez AJ, White CA, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. - PubMed
-
- Forstpointner R, Dreyling M, Repp R, et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2004;104:3064–3071. - PubMed
-
- Howard OM, Gribben JG, Neuberg DS, et al. Rituximab and CHOP induction therapy for newly diagnosed mantle-cell lymphoma: molecular complete responses are not predictive of progression-free survival. J Clin Oncol. 2002;20:1288–1294. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

