Analysis of the synaptotagmin family during reconstituted membrane fusion. Uncovering a class of inhibitory isoforms
- PMID: 18508778
- PMCID: PMC2490792
- DOI: 10.1074/jbc.M709628200
Analysis of the synaptotagmin family during reconstituted membrane fusion. Uncovering a class of inhibitory isoforms
Abstract
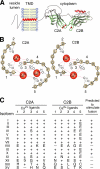
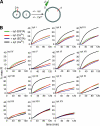
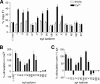
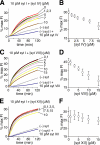
Ca(2+)-triggered exocytosis in neurons and neuroendocrine cells is regulated by the Ca(2+)-binding protein synaptotagmin (syt) I. Sixteen additional isoforms of syt have been identified, but little is known concerning their biochemical or functional properties. Here, we assessed the abilities of fourteen syt isoforms to directly regulate SNARE (soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptor)-catalyzed membrane fusion. One group of isoforms stimulated neuronal SNARE-mediated fusion in response to Ca(2+), while another set inhibited SNARE catalyzed fusion in both the absence and presence of Ca(2+). Biochemical analysis revealed a strong correlation between the ability of syt isoforms to bind 1,2-dioleoyl phosphatidylserine (PS) and t-SNAREs in a Ca(2+)-promoted manner with their abilities to enhance fusion, further establishing PS and SNAREs as critical effectors for syt action. The ability of syt I to efficiently stimulate fusion was specific for certain SNARE pairs, suggesting that syts might contribute to the specificity of intracellular membrane fusion reactions. Finally, a subset of inhibitory syts down-regulated the ability of syt I to activate fusion, demonstrating that syt isoforms can modulate the function of each other.
Figures


Similar articles
-
Regulation of exocytosis and fusion pores by synaptotagmin-effector interactions.Mol Biol Cell. 2010 Aug 15;21(16):2821-31. doi: 10.1091/mbc.E10-04-0285. Epub 2010 Jun 23. Mol Biol Cell. 2010. PMID: 20573977 Free PMC article.
-
Synaptotagmin isoforms couple distinct ranges of Ca2+, Ba2+, and Sr2+ concentration to SNARE-mediated membrane fusion.Mol Biol Cell. 2005 Oct;16(10):4755-64. doi: 10.1091/mbc.e05-04-0277. Epub 2005 Aug 10. Mol Biol Cell. 2005. PMID: 16093350 Free PMC article.
-
Ca(2+)-synaptotagmin directly regulates t-SNARE function during reconstituted membrane fusion.Nat Struct Mol Biol. 2006 Apr;13(4):323-30. doi: 10.1038/nsmb1076. Epub 2006 Mar 26. Nat Struct Mol Biol. 2006. PMID: 16565726
-
Synaptotagmin regulates mast cell functions.Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
-
Function of Drosophila Synaptotagmins in membrane trafficking at synapses.Cell Mol Life Sci. 2021 May;78(9):4335-4364. doi: 10.1007/s00018-021-03788-9. Epub 2021 Feb 22. Cell Mol Life Sci. 2021. PMID: 33619613 Free PMC article. Review.
Cited by
-
A manual collection of Syt, Esyt, Rph3a, Rph3al, Doc2, and Dblc2 genes from 46 metazoan genomes--an open access resource for neuroscience and evolutionary biology.BMC Genomics. 2010 Jan 15;11:37. doi: 10.1186/1471-2164-11-37. BMC Genomics. 2010. PMID: 20078875 Free PMC article.
-
Development of the hypersecretory phenotype in the population of adrenal chromaffin cells from prehypertensive SHRs.Pflugers Arch. 2021 Nov;473(11):1775-1793. doi: 10.1007/s00424-021-02614-2. Epub 2021 Sep 11. Pflugers Arch. 2021. PMID: 34510285
-
Expression and distribution of synaptotagmin family members in the zebrafish retina.J Comp Neurol. 2022 Mar;530(4):705-728. doi: 10.1002/cne.25238. Epub 2021 Sep 24. J Comp Neurol. 2022. PMID: 34468021 Free PMC article.
-
A novel method for culturing stellate astrocytes reveals spatially distinct Ca2+ signaling and vesicle recycling in astrocytic processes.J Gen Physiol. 2017 Jan;149(1):149-170. doi: 10.1085/jgp.201611607. Epub 2016 Dec 1. J Gen Physiol. 2017. PMID: 27908976 Free PMC article.
-
Axonal and dendritic synaptotagmin isoforms revealed by a pHluorin-syt functional screen.Mol Biol Cell. 2012 May;23(9):1715-27. doi: 10.1091/mbc.E11-08-0707. Epub 2012 Mar 7. Mol Biol Cell. 2012. PMID: 22398727 Free PMC article.
References
-
- Niemann, H., Blasi, J., and Jahn, R. (1994) Trends Cell Biol. 4 179-185 - PubMed
-
- Jahn, R., and Scheller, R. H. (2006) Nat. Rev. Mol. Cell Biol. 7 631-643 - PubMed
-
- Weber, T., Zemelman, B. V., McNew, J. A., Westermann, B., Gmachl, M., Parlati, F., Sollner, T. H., and Rothman, J. E. (1998) Cell 92 759-772 - PubMed
-
- Augustine, G. J. (2001) Curr. Opin. Neurobiol. 11 320-326 - PubMed
-
- Koh, T. W., and Bellen, H. J. (2003) Trends Neurosci. 26 413-422 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

