Hematopoietic cell transplantation provides an immune-tolerant platform for myoblast transplantation in dystrophic dogs
- PMID: 18500253
- PMCID: PMC2536604
- DOI: 10.1038/mt.2008.102
Hematopoietic cell transplantation provides an immune-tolerant platform for myoblast transplantation in dystrophic dogs
Abstract
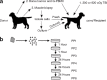
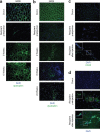
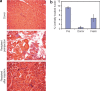
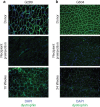
Duchenne Muscular Dystrophy (DMD) is the most common and severe form of muscular dystrophy in humans. The goal of myogenic stem cell transplant therapy for DMD is to increase dystrophin expression in existing muscle fibers and to provide a source of stem cells for future muscle generation. Although syngeneic myogenic stem cell transplants have been successful in mice, allogeneic transplants of myogenic stem cells were ineffective in several human trials. To determine whether allogeneic muscle progenitor cells can be successfully transplanted in an immune-tolerant recipient, we induced immune tolerance in two DMD-affected (cxmd) dogs through hematopoietic cell transplantation (HCT). Injection of freshly isolated muscle-derived cells from the HCT donor into either fully or partially chimeric xmd recipients restored dystrophin expression up to 6.48% of wild-type levels, reduced the number of centrally located nuclei, and improved muscle structure. Dystrophin expression was maintained for at least 24 weeks. Taken together, these data indicate that immune tolerance to donor myoblasts provides an important platform from which to further improve myoblast transplantation, with the goal of restoring dystrophin expression to patients with DMD.
Figures
Similar articles
-
Hematopoietic stem cell transplantation does not restore dystrophin expression in Duchenne muscular dystrophy dogs.Blood. 2004 Dec 15;104(13):4311-8. doi: 10.1182/blood-2004-06-2247. Epub 2004 Aug 24. Blood. 2004. PMID: 15328150
-
Induction of CCAAT/Enhancer-Binding Protein β Expression With the Phosphodiesterase Inhibitor Isobutylmethylxanthine Improves Myoblast Engraftment Into Dystrophic Muscle.Stem Cells Transl Med. 2016 Apr;5(4):500-10. doi: 10.5966/sctm.2015-0169. Epub 2016 Mar 3. Stem Cells Transl Med. 2016. PMID: 26941360 Free PMC article.
-
Creation of Dystrophin Expressing Chimeric Cells of Myoblast Origin as a Novel Stem Cell Based Therapy for Duchenne Muscular Dystrophy.Stem Cell Rev Rep. 2018 Apr;14(2):189-199. doi: 10.1007/s12015-017-9792-7. Stem Cell Rev Rep. 2018. PMID: 29305755 Free PMC article.
-
[Transplantation of normal or genetically modified myoblasts for the treatment of hereditary or acquired diseases].J Soc Biol. 2001;195(1):29-37. J Soc Biol. 2001. PMID: 11530497 Review. French.
-
Stem cell-based therapies for Duchenne muscular dystrophy.Exp Neurol. 2020 Jan;323:113086. doi: 10.1016/j.expneurol.2019.113086. Epub 2019 Oct 19. Exp Neurol. 2020. PMID: 31639376 Free PMC article. Review.
Cited by
-
Generation and characterization of integration-free induced pluripotent stem cells from patients with autoimmune disease.Exp Mol Med. 2016 May 13;48(5):e232. doi: 10.1038/emm.2016.27. Exp Mol Med. 2016. PMID: 27174201 Free PMC article.
-
Decoding the Gene Regulatory Network of Muscle Stem Cells in Mouse Duchenne Muscular Dystrophy: Revelations from Single-Nuclei RNA Sequencing Analysis.Int J Mol Sci. 2023 Aug 5;24(15):12463. doi: 10.3390/ijms241512463. Int J Mol Sci. 2023. PMID: 37569835 Free PMC article.
-
Successful bone marrow transplantation in a patient with Diamond-Blackfan anemia with co-existing Duchenne muscular dystrophy: a case report.J Med Case Rep. 2011 Jun 4;5:216. doi: 10.1186/1752-1947-5-216. J Med Case Rep. 2011. PMID: 21639928 Free PMC article.
-
Chronic administration of membrane sealant prevents severe cardiac injury and ventricular dilatation in dystrophic dogs.J Clin Invest. 2010 Apr;120(4):1140-50. doi: 10.1172/JCI41329. Epub 2010 Mar 15. J Clin Invest. 2010. PMID: 20234088 Free PMC article.
-
Gene therapy in large animal models of muscular dystrophy.ILAR J. 2009;50(2):187-98. doi: 10.1093/ilar.50.2.187. ILAR J. 2009. PMID: 19293461 Free PMC article. Review.
References
-
- Chakkalakal JV, Thompson J, Parks RJ, Jasmin BJ. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies (review) FASEB J. 2005;19:880–891. - PubMed
-
- Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–179. - PubMed
Publication types
MeSH terms
Grants and funding
- P01-NS046788/NS/NINDS NIH HHS/United States
- U54 HD047175-05/HD/NICHD NIH HHS/United States
- U54 HD047175/HD/NICHD NIH HHS/United States
- CA15704/CA/NCI NIH HHS/United States
- P30 DK056465/DK/NIDDK NIH HHS/United States
- P01 NS046788/NS/NINDS NIH HHS/United States
- DK56465/DK/NIDDK NIH HHS/United States
- P30 CA015704-34/CA/NCI NIH HHS/United States
- P01 CA078902/CA/NCI NIH HHS/United States
- P01 CA078902-10/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- CA78902/CA/NCI NIH HHS/United States
- P30 DK056465-09/DK/NIDDK NIH HHS/United States
- HD47175/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources

