Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth
- PMID: 18498733
- PMCID: PMC2448395
- DOI: 10.1016/j.neuron.2008.03.007
Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth
Abstract
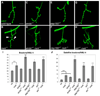
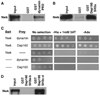
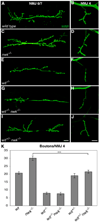
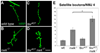
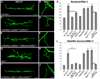
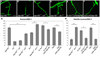
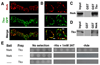
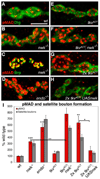
Regulation of synaptic growth is fundamental to the formation and plasticity of neural circuits. Here, we demonstrate that Nervous wreck (Nwk), a negative regulator of synaptic growth at Drosophila NMJs, interacts functionally and physically with components of the endocytic machinery, including dynamin and Dap160/intersectin, and negatively regulates retrograde BMP growth signaling through a direct interaction with the BMP receptor, thickveins. Synaptic overgrowth in nwk is sensitive to BMP signaling levels, and loss of Nwk facilitates BMP-induced overgrowth. Conversely, Nwk overexpression suppresses BMP-induced synaptic overgrowth. We observe analogous genetic interactions between dap160 and the BMP pathway, confirming that endocytosis regulates BMP signaling at NMJs. Finally, we demonstrate a correlation between synaptic growth and pMAD levels and show that Nwk regulates these levels. We propose that Nwk functions at the interface of endocytosis and BMP signaling to ensure proper synaptic growth by negatively regulating Tkv to set limits on this positive growth signal.
Figures
Similar articles
-
Neuroligin 4 regulates synaptic growth via the bone morphogenetic protein (BMP) signaling pathway at the Drosophila neuromuscular junction.J Biol Chem. 2017 Nov 3;292(44):17991-18005. doi: 10.1074/jbc.M117.810242. Epub 2017 Sep 14. J Biol Chem. 2017. PMID: 28912273 Free PMC article.
-
Drosophila S6 Kinase like inhibits neuromuscular junction growth by downregulating the BMP receptor thickveins.PLoS Genet. 2015 Mar 6;11(3):e1004984. doi: 10.1371/journal.pgen.1004984. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25748449 Free PMC article.
-
Nervous wreck and Cdc42 cooperate to regulate endocytic actin assembly during synaptic growth.J Neurosci. 2008 Aug 13;28(33):8316-25. doi: 10.1523/JNEUROSCI.2304-08.2008. J Neurosci. 2008. PMID: 18701694 Free PMC article.
-
[MVam2-dependent endocytic pathway regulates BMP signaling during mouse early development].Seikagaku. 2013 Sep;85(9):806-9. Seikagaku. 2013. PMID: 24195314 Review. Japanese. No abstract available.
-
Synapse scaffolding: intersection of endocytosis and growth.Curr Biol. 2004 Oct 5;14(19):R853-5. doi: 10.1016/j.cub.2004.09.042. Curr Biol. 2004. PMID: 15458667 Review.
Cited by
-
AP2 Regulates Thickveins Trafficking to Attenuate NMJ Growth Signaling in Drosophila.eNeuro. 2022 Oct 12;9(5):ENEURO.0044-22.2022. doi: 10.1523/ENEURO.0044-22.2022. Print 2022 Sep-Oct. eNeuro. 2022. PMID: 36180220 Free PMC article.
-
New approaches for studying synaptic development, function, and plasticity using Drosophila as a model system.J Neurosci. 2013 Nov 6;33(45):17560-8. doi: 10.1523/JNEUROSCI.3261-13.2013. J Neurosci. 2013. PMID: 24198346 Free PMC article. Review.
-
Membrane Charge Directs the Outcome of F-BAR Domain Lipid Binding and Autoregulation.Cell Rep. 2015 Dec 22;13(11):2597-2609. doi: 10.1016/j.celrep.2015.11.044. Epub 2015 Dec 10. Cell Rep. 2015. PMID: 26686642 Free PMC article.
-
The translational regulator Cup controls NMJ presynaptic terminal morphology.Mol Cell Neurosci. 2015 Jul;67:126-36. doi: 10.1016/j.mcn.2015.06.010. Epub 2015 Jun 20. Mol Cell Neurosci. 2015. PMID: 26102195 Free PMC article.
-
The calcium channel subunit α2δ-3 organizes synapses via an activity-dependent and autocrine BMP signaling pathway.Nat Commun. 2019 Dec 6;10(1):5575. doi: 10.1038/s41467-019-13165-7. Nat Commun. 2019. PMID: 31811118 Free PMC article.
References
-
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron. 2002;33:545–558. - PubMed
-
- Allan DW, St Pierre SE, Miguel-Aliaga I, Thor S. Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell. 2003;113:73–86. - PubMed
-
- Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science (New York, N.Y. 2006;314:1135–1139. - PubMed
-
- Chen Y, Riese MJ, Killinger MA, Hoffmann FM. A genetic screen for modifiers of Drosophila decapentaplegic signaling identifies mutations in punt, Mothers against dpp and the BMP-7 homologue, 60A. Development (Cambridge, England) 1998;125:1759–1768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

