Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function
- PMID: 18495876
- PMCID: PMC2633923
- DOI: 10.1523/JNEUROSCI.0955-08.2008
Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function
Abstract
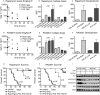
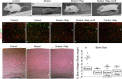
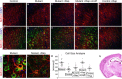
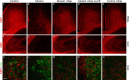
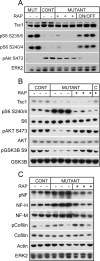
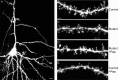
Tuberous sclerosis (TSC) is a hamartoma syndrome attributable to mutations in either TSC1 or TSC2 in which brain involvement causes epilepsy, mental retardation, and autism. We have reported recently (Meikle et al., 2007) a mouse neuronal model of TSC in which Tsc1 is ablated in most neurons during cortical development. We have tested rapamycin and RAD001 [40-O-(2-hydroxyethyl)-rapamycin], both mammalian target of rapamycin mTORC1 inhibitors, as potential therapeutic agents in this model. Median survival is improved from 33 d to more than 100 d; behavior, phenotype, and weight gain are all also markedly improved. There is brain penetration of both drugs, with accumulation over time with repetitive treatment, and effective reduction of levels of phospho-S6, a downstream target of mTORC1. In addition, there is restoration of phospho-Akt and phospho-glycogen synthase kinase 3 levels in the treated mice, consistent with restoration of Akt function. Neurofilament abnormalities, myelination, and cell enlargement are all improved by the treatment. However, dysplastic neuronal features persist, and there are only modest changes in dendritic spine density and length. Strikingly, mice treated with rapamycin or RAD001 for 23 d only (postnatal days 7-30) displayed a persistent improvement in phenotype, with median survival of 78 d. In summary, rapamycin/RAD001 are highly effective therapies for this neuronal model of TSC, with benefit apparently attributable to effects on mTORC1 and Akt signaling and, consequently, cell size and myelination. Although caution is appropriate, the results suggest the possibility that rapamycin/RAD001 may have benefit in the treatment of TSC brain disease, including infantile spasms.
Figures
Comment in
-
"TOR"rents of excitement over rapamycin's antiepileptogenic potential.Epilepsy Curr. 2008 Nov-Dec;8(6):163-5. doi: 10.1111/j.1535-7511.2008.00280.x. Epilepsy Curr. 2008. PMID: 19127312 Free PMC article. No abstract available.
Similar articles
-
Equivalent benefit of mTORC1 blockade and combined PI3K-mTOR blockade in a mouse model of tuberous sclerosis.Mol Cancer. 2009 Jun 15;8:38. doi: 10.1186/1476-4598-8-38. Mol Cancer. 2009. PMID: 19527517 Free PMC article.
-
Neuronal and glia abnormalities in Tsc1-deficient forebrain and partial rescue by rapamycin.Neurobiol Dis. 2012 Jan;45(1):369-80. doi: 10.1016/j.nbd.2011.08.024. Epub 2011 Aug 26. Neurobiol Dis. 2012. PMID: 21907282 Free PMC article.
-
Rapamycin weekly maintenance dosing and the potential efficacy of combination sorafenib plus rapamycin but not atorvastatin or doxycycline in tuberous sclerosis preclinical models.BMC Pharmacol. 2009 Apr 15;9:8. doi: 10.1186/1471-2210-9-8. BMC Pharmacol. 2009. PMID: 19368729 Free PMC article.
-
Mammalian target of rapamycin and tuberous sclerosis complex.J Dermatol Sci. 2015 Aug;79(2):93-100. doi: 10.1016/j.jdermsci.2015.04.005. Epub 2015 Apr 25. J Dermatol Sci. 2015. PMID: 26051878 Review.
-
Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex.Neuro Oncol. 2015 Dec;17(12):1550-9. doi: 10.1093/neuonc/nov152. Epub 2015 Aug 19. Neuro Oncol. 2015. PMID: 26289591 Free PMC article. Review.
Cited by
-
Prenatal rapamycin results in early and late behavioral abnormalities in wildtype C57BL/6 mice.Behav Genet. 2013 Jan;43(1):51-9. doi: 10.1007/s10519-012-9571-9. Epub 2012 Dec 12. Behav Genet. 2013. PMID: 23229624 Free PMC article.
-
Diet and energy-sensing inputs affect TorC1-mediated axon misrouting but not TorC2-directed synapse growth in a Drosophila model of tuberous sclerosis.PLoS One. 2012;7(2):e30722. doi: 10.1371/journal.pone.0030722. Epub 2012 Feb 3. PLoS One. 2012. PMID: 22319582 Free PMC article.
-
From mTOR to cognition: molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis.J Intellect Disabil Res. 2009 Oct;53(10):838-51. doi: 10.1111/j.1365-2788.2009.01208.x. Epub 2009 Aug 19. J Intellect Disabil Res. 2009. PMID: 19694899 Free PMC article. Review.
-
Targeting protein kinases in central nervous system disorders.Nat Rev Drug Discov. 2009 Nov;8(11):892-909. doi: 10.1038/nrd2999. Nat Rev Drug Discov. 2009. PMID: 19876042 Free PMC article. Review.
-
Imbalanced pattern completion vs. separation in cognitive disease: network simulations of synaptic pathologies predict a personalized therapeutics strategy.BMC Neurosci. 2010 Aug 13;11:96. doi: 10.1186/1471-2202-11-96. BMC Neurosci. 2010. PMID: 20704756 Free PMC article.
References
-
- Au KS, Williams AT, Roach ES, Batchelor L, Sparagana SP, Delgado MR, Wheless JW, Baumgartner JE, Roa BB, Wilson CM, Smith-Knuppel TK, Cheung MY, Whittemore VH, King TM, Northrup H. Genotype/phenotype correlation in 325 individuals referred for a diagnosis of tuberous sclerosis complex in the United States. Genet Med. 2007;9:88–100. - PubMed
-
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. - PubMed
-
- Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G, II, Aronica E, Crino PB. mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol. 2004;56:478–487. - PubMed
-
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
