Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination
- PMID: 18495775
- PMCID: PMC2493346
- DOI: 10.1128/JVI.00562-08
Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination
Abstract
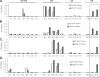
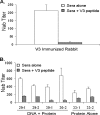
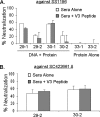
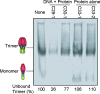
A major challenge in human immunodeficiency virus type 1 (HIV-1) vaccine development is to elicit potent and broadly neutralizing antibodies that are effective against primary viral isolates. Previously, we showed that DNA prime-protein boost vaccination using HIV-1 gp120 antigens was more effective in eliciting neutralizing antibodies against primary HIV-1 isolates than was a recombinant gp120 protein-only vaccination approach. In the current study, we analyzed the difference in antibody specificities in rabbit sera elicited by these two immunization regimens using peptide enzyme-linked immunosorbent assay and a competitive virus capture assay. Our results indicate that a DNA prime-protein boost regimen is more effective than a protein-alone vaccination approach in inducing antibodies that target two key neutralizing domains: the V3 loop and the CD4 binding site. In particular, positive antibodies targeting several peptides that overlap with the known CD4 binding area were detected only in DNA-primed sera. Different profiles of antibody specificities provide insight into the mechanisms behind the elicitation of better neutralizing antibodies with the DNA prime-protein boost approach, and our results support the use of this approach to further optimize Env formulations for HIV vaccine development.
Figures



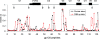

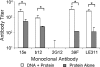
Similar articles
-
Induction of Heterologous Tier 2 HIV-1-Neutralizing and Cross-Reactive V1/V2-Specific Antibodies in Rabbits by Prime-Boost Immunization.J Virol. 2016 Sep 12;90(19):8644-60. doi: 10.1128/JVI.00853-16. Print 2016 Oct 1. J Virol. 2016. PMID: 27440894 Free PMC article.
-
Membrane bound Indian clade C HIV-1 envelope antigen induces antibodies to diverse and conserved epitopes upon DNA prime/protein boost in rabbits.Vaccine. 2016 May 5;34(21):2444-2452. doi: 10.1016/j.vaccine.2016.03.062. Epub 2016 Mar 28. Vaccine. 2016. PMID: 27032514
-
DNA prime-protein boost using subtype consensus Env was effective in eliciting neutralizing antibody responses against subtype BC HIV-1 viruses circulating in China.Hum Vaccin Immunother. 2012 Nov 1;8(11):1630-7. doi: 10.4161/hv.21648. Epub 2012 Oct 30. Hum Vaccin Immunother. 2012. PMID: 23111170 Free PMC article.
-
A Trimeric HIV-1 Envelope gp120 Immunogen Induces Potent and Broad Anti-V1V2 Loop Antibodies against HIV-1 in Rabbits and Rhesus Macaques.J Virol. 2018 Feb 12;92(5):e01796-17. doi: 10.1128/JVI.01796-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237847 Free PMC article.
-
Development of an oral prime-boost strategy to elicit broadly neutralizing antibodies against HIV-1.Vaccine. 2002 May 6;20(15):1968-74. doi: 10.1016/s0264-410x(02)00080-4. Vaccine. 2002. PMID: 11983256 Review.
Cited by
-
Co-immunization with multimeric scaffolds and DNA rapidly induces potent autologous HIV-1 neutralizing antibodies and CD8+ T cells.PLoS One. 2012;7(2):e31464. doi: 10.1371/journal.pone.0031464. Epub 2012 Feb 16. PLoS One. 2012. PMID: 22359593 Free PMC article.
-
Two closely related Env antigens from the same patient elicited different spectra of neutralizing antibodies against heterologous HIV-1 isolates.J Virol. 2011 May;85(10):4927-36. doi: 10.1128/JVI.00081-11. Epub 2011 Mar 16. J Virol. 2011. PMID: 21411542 Free PMC article.
-
Mucosal vaccines against respiratory syncytial virus.Curr Opin Virol. 2014 Jun;6:78-84. doi: 10.1016/j.coviro.2014.03.009. Epub 2014 Apr 29. Curr Opin Virol. 2014. PMID: 24794644 Free PMC article. Review.
-
Structure-based Design of Cyclically Permuted HIV-1 gp120 Trimers That Elicit Neutralizing Antibodies.J Biol Chem. 2017 Jan 6;292(1):278-291. doi: 10.1074/jbc.M116.725614. Epub 2016 Nov 22. J Biol Chem. 2017. PMID: 27879316 Free PMC article.
-
Harnessing early life immunity to develop a pediatric HIV vaccine that can protect through adolescence.PLoS Pathog. 2020 Nov 12;16(11):e1008983. doi: 10.1371/journal.ppat.1008983. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33180867 Free PMC article. No abstract available.
References
-
- Beddows, S., N. Schulke, M. Kirschner, K. Barnes, M. Franti, E. Michael, T. Ketas, R. W. Sanders, P. J. Maddon, W. C. Olson, and J. P. Moore. 2005. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 798812-8827. - PMC - PubMed
-
- Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 7813232-13252. - PMC - PubMed
-
- Conley, A. J., J. A. Kessler II, L. J. Boots, P. M. McKenna, W. A. Schleif, E. A. Emini, G. E. Mark III, H. Katinger, E. K. Cobb, S. M. Lunceford, S. R. Rouse, and K. K. Murthy. 1996. The consequence of passive administration of an anti-human immunodeficiency virus type 1 neutralizing monoclonal antibody before challenge of chimpanzees with a primary virus isolate. J. Virol. 706751-6758. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

