Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line
- PMID: 18492291
- PMCID: PMC2412912
- DOI: 10.1186/1476-4598-7-42
Evidence for efficient phosphorylation of EGFR and rapid endocytosis of phosphorylated EGFR via the early/late endocytic pathway in a gefitinib-sensitive non-small cell lung cancer cell line
Abstract
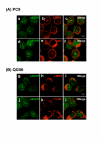
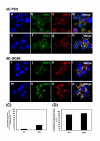
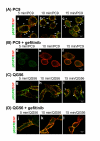
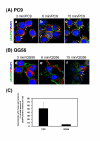
Gefitinib (Iressa)-a specific inhibitor of epidermal growth factor receptor (EGFR) tyrosine kinase-has been shown to suppress the activation of EGFR signaling required for cell survival and proliferation in non-small cell lung cancer (NSCLC) cell lines. We recently provided novel evidence that gefitinib-sensitive PC9 cells show normal endocytosis of EGFR: internalized EGF-EGFR complexes were transported to late endosomes/lysosomes 15 min after EGF stimulation, and then degraded within the lysosomes. However, gefitinib-resistant QG56 cells showed internalized EGFR accumulation in early endosomes after 60 min of internalization, instead of its trafficking to lysosomes, indicating an aberration in some steps of EGF-EGFR trafficking from the early endosomes to late endosomes/lysosomes. Therefore, we postulate that impairment in some steps of EGF-EGFR trafficking from early endosomes to late endosomes/lysosomes might confer gefitinib-resistance in NSCLC cell lines. To further substantiate the detailed internalization mechanism of gefitinib-sensitive and gefitinib-resistant cells, using confocal immunofluorescence microscopy, we examined the endocytic trafficking of phosphorylated EGFR (pEGFR) in the absence or presence of gefitinib. In PC9 and QG56 cells without EGF stimulation, a large number of pEGFR-positive small vesicular structures not colocalized with late endosomes/lysosomes were spread throughout the cytoplasm, and some pEGFR staining was distributed in the nucleus. This implies a novel intracellular trafficking pathway for pEGFR from cytoplasmic vesicles to the nucleus. Furthermore, an aggregated vesicular structure of early endosomes was observed in the perinuclear region of QG56 cells; it was revealed to be associated with SNX1, originally identified as a protein that interacts with EGFR. Therefore, we confirmed our previous data that an aberration in some steps of EGF-EGFR trafficking from the early endosomes to late endosomes/lysosomes occurs in QG56 cells. Furthermore, in PC9 cells, efficient phosphorylation of EGFR and rapid internalization of pEGFR was observed at 3 min after EGF stimulation; these internalized pEGFR-positive vesicles were trafficked to late endosomes at 15 min, indicating rapid trafficking of EGF-pEGFR complexes from early to late endosomes in PC9 cells. Gefitinib treatment strongly reduced the phosphorylation level of EGFR, and subsequent endocytosis of EGFR was significantly suppressed in PC9 cells. In contrast, in QG56 cells, EGFR trafficking via the early endocytic pathway was basically impaired; therefore, gefitinib appeared to slightly suppress the internalization of pEGFR. Collectively, our data provide novel evidence that extensive impairment in pEGFR endocytosis via the early endocytic pathway might confer gefitinib-resistance in QG56 cells.
Figures
Similar articles
-
Involvement of SNX1 in regulating EGFR endocytosis in a gefitinib-resistant NSCLC cell lines.Cancer Drug Resist. 2019 Sep 19;2(3):539-549. doi: 10.20517/cdr.2019.15. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582586 Free PMC article. Review.
-
The EGFR inhibitor gefitinib suppresses ligand-stimulated endocytosis of EGFR via the early/late endocytic pathway in non-small cell lung cancer cell lines.Histochem Cell Biol. 2007 May;127(5):541-53. doi: 10.1007/s00418-007-0281-y. Epub 2007 Mar 15. Histochem Cell Biol. 2007. PMID: 17361439
-
Silencing of SNX1 by siRNA stimulates the ligand-induced endocytosis of EGFR and increases EGFR phosphorylation in gefitinib-resistant human lung cancer cell lines.Int J Oncol. 2012 Oct;41(4):1520-30. doi: 10.3892/ijo.2012.1578. Epub 2012 Jul 31. Int J Oncol. 2012. PMID: 22859339
-
EGF‑stimulated AKT activation is mediated by EGFR recycling via an early endocytic pathway in a gefitinib‑resistant human lung cancer cell line.Int J Oncol. 2015 Apr;46(4):1721-9. doi: 10.3892/ijo.2015.2871. Epub 2015 Feb 4. Int J Oncol. 2015. PMID: 25653196
-
Endocytosis and intracellular trafficking of ErbBs.Exp Cell Res. 2008 Oct 15;314(17):3093-106. doi: 10.1016/j.yexcr.2008.08.013. Epub 2008 Aug 28. Exp Cell Res. 2008. PMID: 18793634 Free PMC article. Review.
Cited by
-
Involvement of SNX1 in regulating EGFR endocytosis in a gefitinib-resistant NSCLC cell lines.Cancer Drug Resist. 2019 Sep 19;2(3):539-549. doi: 10.20517/cdr.2019.15. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582586 Free PMC article. Review.
-
EGFR targeted therapies and radiation: Optimizing efficacy by appropriate drug scheduling and patient selection.Pharmacol Ther. 2015 Oct;154:67-77. doi: 10.1016/j.pharmthera.2015.07.002. Epub 2015 Jul 21. Pharmacol Ther. 2015. PMID: 26205191 Free PMC article. Review.
-
EGFR phosphorylates HDAC1 to regulate its expression and anti-apoptotic function.Cell Death Dis. 2021 May 11;12(5):469. doi: 10.1038/s41419-021-03697-6. Cell Death Dis. 2021. PMID: 33976119 Free PMC article.
-
Identification of a novel endocytosis‑associated gene signature for prognostic prediction in lung adenocarcinoma.Oncol Lett. 2023 Oct 12;26(6):511. doi: 10.3892/ol.2023.14098. eCollection 2023 Dec. Oncol Lett. 2023. PMID: 37920434 Free PMC article.
-
Genetic Engineering of Bacteriophage K1F with Human Epidermal Growth Factor to Enhance Killing of Intracellular E. coli K1.ACS Synth Biol. 2023 Jul 21;12(7):2094-2106. doi: 10.1021/acssynbio.3c00135. Epub 2023 Jun 15. ACS Synth Biol. 2023. PMID: 37318278 Free PMC article.
References
-
- Carpenter G, Cohen S. Epidermal growth factor. J Biol Chem. 1990;265:7709–7712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

