Structure of TRPV1 channel revealed by electron cryomicroscopy
- PMID: 18490661
- PMCID: PMC2396679
- DOI: 10.1073/pnas.0711835105
Structure of TRPV1 channel revealed by electron cryomicroscopy
Abstract
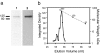
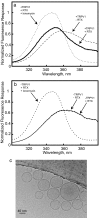
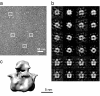
The transient receptor potential (TRP) family of ion channels participate in many signaling pathways. TRPV1 functions as a molecular integrator of noxious stimuli, including heat, low pH, and chemical ligands. Here, we report the 3D structure of full-length rat TRPV1 channel expressed in the yeast Saccharomyces cerevisiae and purified by immunoaffinity chromatography. We demonstrate that the recombinant purified TRPV1 channel retains its structural and functional integrity and is suitable for structural analysis. The 19-A structure of TRPV1 determined by using single-particle electron cryomicroscopy exhibits fourfold symmetry and comprises two distinct regions: a large open basket-like domain, likely corresponding to the cytoplasmic N- and C-terminal portions, and a more compact domain, corresponding to the transmembrane portion. The assignment of transmembrane and cytoplasmic regions was supported by fitting crystal structures of the structurally homologous Kv1.2 channel and isolated TRPV1 ankyrin repeats into the TRPV1 structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

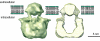

Similar articles
-
Toward elucidating the heat activation mechanism of the TRPV1 channel gating by molecular dynamics simulation.Proteins. 2016 Dec;84(12):1938-1949. doi: 10.1002/prot.25177. Epub 2016 Oct 24. Proteins. 2016. PMID: 27699868 Free PMC article.
-
Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states.J Membr Biol. 2008 Jun;223(3):161-72. doi: 10.1007/s00232-008-9123-7. Epub 2008 Sep 14. J Membr Biol. 2008. PMID: 18791833
-
Structure of the TRPV1 ion channel determined by electron cryo-microscopy.Nature. 2013 Dec 5;504(7478):107-12. doi: 10.1038/nature12822. Nature. 2013. PMID: 24305160 Free PMC article.
-
Determining the Crystal Structure of TRPV6.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 14. PMID: 30299652 Free Books & Documents. Review.
-
Single particle electron cryo-microscopy of a mammalian ion channel.Curr Opin Struct Biol. 2014 Aug;27:1-7. doi: 10.1016/j.sbi.2014.02.005. Epub 2014 Mar 25. Curr Opin Struct Biol. 2014. PMID: 24681231 Free PMC article. Review.
Cited by
-
Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch.Int J Mol Sci. 2020 Jul 10;21(14):4883. doi: 10.3390/ijms21144883. Int J Mol Sci. 2020. PMID: 32664385 Free PMC article. Review.
-
Toward elucidating the heat activation mechanism of the TRPV1 channel gating by molecular dynamics simulation.Proteins. 2016 Dec;84(12):1938-1949. doi: 10.1002/prot.25177. Epub 2016 Oct 24. Proteins. 2016. PMID: 27699868 Free PMC article.
-
TRPM6 N-Terminal CaM- and S100A1-Binding Domains.Int J Mol Sci. 2019 Sep 9;20(18):4430. doi: 10.3390/ijms20184430. Int J Mol Sci. 2019. PMID: 31505788 Free PMC article.
-
Divalent cations potentiate TRPV1 channel by lowering the heat activation threshold.J Gen Physiol. 2014 Jan;143(1):75-90. doi: 10.1085/jgp.201311025. Epub 2013 Dec 16. J Gen Physiol. 2014. PMID: 24344247 Free PMC article.
-
Structural insights into the gating mechanisms of TRPV channels.Cell Calcium. 2020 May;87:102168. doi: 10.1016/j.ceca.2020.102168. Epub 2020 Jan 24. Cell Calcium. 2020. PMID: 32004816 Free PMC article. Review.
References
-
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. - PubMed
-
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: A putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. - PubMed
-
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;272:re3. - PubMed
-
- Nilius B, Voets T. TRP channels: A TR(I)P through a world of multifunctional cation channels. Pflügers Arch. 2005;451:1–10. - PubMed
-
- Voets T, Talavera K, Owsianik G, Nilius B. Sensing with TRP channels. Nat Chem Biol. 2005;1:85–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

