In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions
- PMID: 18480441
- PMCID: PMC2446952
- DOI: 10.1128/JVI.00697-08
In vitro-generated antigen-specific CD4+ CD25+ Foxp3+ regulatory T cells control the severity of herpes simplex virus-induced ocular immunoinflammatory lesions
Abstract
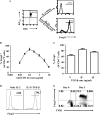
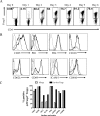
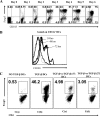
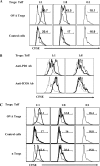
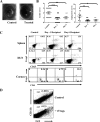
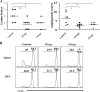
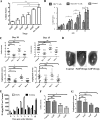
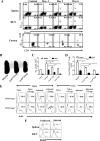
Generating and using regulatory T cells (Tregs) to modulate inflammatory disease represents a valuable approach to therapy but has not yet been applied as a means to control virus-induced immunopathological reactions. In this report, we developed a simplified technique that used unfractionated splenocytes as a precursor population and showed that stimulation under optimized conditions for 5 days with solid-phase anti-CD3 monoclonal antibody in the presence of transforming growth factor beta (TGF-beta) and interleukin-2 could induce up to 90% of CD4(+) T cells to become Foxp3(+) and able to mediate suppression in vitro. CD11c(+) dendritic cells were intricately involved in the conversion process and, once modified in the presence of TGF-beta, could convert Foxp3(-) CD4(+) cells into Foxp3(+) CD4(+)cells by producing TGF-beta. The converted cells had undergone cell division, and the majority of them expressed activation markers along with surface molecules that would facilitate their migration into tissue sites. The primary reason for our study was to determine if such in vitro-converted Tregs could be used in vivo to influence the outcome of a virus-induced immunoinflammatory lesion in the eye caused by herpes simplex virus infection. We could show in three separate models of herpetic stromal keratitis that adoptive transfers of in vitro-converted Tregs effectively diminished lesion severity, especially when given in the initial phases of infection. The suppression effect in vivo appeared to be polyspecific. The protocol we have developed could provide a useful additional approach to control virus-induced inflammatory disease.
Figures
Similar articles
-
The Plasticity and Stability of Regulatory T Cells during Viral-Induced Inflammatory Lesions.J Immunol. 2017 Aug 15;199(4):1342-1352. doi: 10.4049/jimmunol.1700520. Epub 2017 Jul 14. J Immunol. 2017. PMID: 28710254 Free PMC article.
-
Anti-inflammatory effects of FTY720 against viral-induced immunopathology: role of drug-induced conversion of T cells to become Foxp3+ regulators.J Immunol. 2008 Jun 1;180(11):7636-47. doi: 10.4049/jimmunol.180.11.7636. J Immunol. 2008. PMID: 18490766
-
CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions.J Immunol. 2004 Apr 1;172(7):4123-32. doi: 10.4049/jimmunol.172.7.4123. J Immunol. 2004. PMID: 15034024
-
The Role of T Cells in Herpes Stromal Keratitis.Front Immunol. 2019 Mar 19;10:512. doi: 10.3389/fimmu.2019.00512. eCollection 2019. Front Immunol. 2019. PMID: 30941142 Free PMC article. Review.
-
Dendritic cells expand antigen-specific Foxp3+ CD25+ CD4+ regulatory T cells including suppressors of alloreactivity.Immunol Rev. 2006 Aug;212:314-29. doi: 10.1111/j.0105-2896.2006.00422.x. Immunol Rev. 2006. PMID: 16903923 Review.
Cited by
-
Suppression of innate immune pathology by regulatory T cells during Influenza A virus infection of immunodeficient mice.J Virol. 2010 Dec;84(24):12564-75. doi: 10.1128/JVI.01559-10. Epub 2010 Oct 13. J Virol. 2010. PMID: 20943986 Free PMC article.
-
CD8+ Treg cells suppress CD8+ T cell-responses by IL-10-dependent mechanism during H5N1 influenza virus infection.Eur J Immunol. 2014 Jan;44(1):103-14. doi: 10.1002/eji.201343583. Epub 2013 Nov 4. Eur J Immunol. 2014. PMID: 24114149 Free PMC article.
-
In vitro induced regulatory T cells are unique from endogenous regulatory T cells and effective at suppressing late stages of ongoing autoimmunity.PLoS One. 2014 Aug 13;9(8):e104698. doi: 10.1371/journal.pone.0104698. eCollection 2014. PLoS One. 2014. PMID: 25119105 Free PMC article.
-
Controlling herpetic stromal keratitis by modulating lymphotoxin-alpha-mediated inflammatory pathways.Microbes Infect. 2013 Sep-Oct;15(10-11):677-87. doi: 10.1016/j.micinf.2013.07.001. Epub 2013 Jul 10. Microbes Infect. 2013. PMID: 23850656 Free PMC article.
-
Regulatory T cells are essential to promote proper CD4 T-cell priming upon mucosal infection.Mucosal Immunol. 2016 Nov;9(6):1395-1406. doi: 10.1038/mi.2016.19. Epub 2016 Mar 23. Mucosal Immunol. 2016. PMID: 27007674 Free PMC article.
References
-
- Battaglia, M., A. Stabilini, B. Migliavacca, J. Horejs-Hoeck, T. Kaupper, and M. G. Roncarolo. 2006. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J. Immunol. 1778338-8347. - PubMed
-
- Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441235-238. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

