The PCH family member proline-serine-threonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration
- PMID: 18480402
- PMCID: PMC2488309
- DOI: 10.1091/mbc.e08-02-0225
The PCH family member proline-serine-threonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration
Abstract
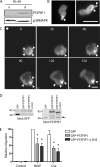
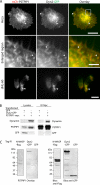
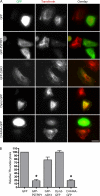
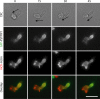
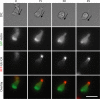
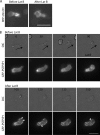
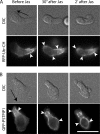
Pombe Cdc15 homology (PCH) family members have emerged as important regulators of membrane-cytoskeletal interactions. Here we show that PSTPIP1, a PCH family member expressed in hematopoietic cells, regulates the motility of neutrophil-like cells and is a novel component of the leukocyte uropod where it colocalizes with other uropod components, such as type I PIPKIgamma. Furthermore, we show that PSTPIP1 association with the regulator of endocytosis, dynamin 2, and PSTPIP1 expression impairs transferrin uptake and endocytosis. We also show that PSTPIP1 localizes at the rear of neutrophils with a subpopulation of F-actin that is specifically detected by the binding of an F-actin probe that detects a more stable population of actin. Finally, we show that actin polymerization, but not the microtubule network, is necessary for the polarized distribution of PSTPIP1 toward the rear of the cell. Together, our findings demonstrate that PSTPIP1 is a novel component of the leukocyte uropod that regulates endocytosis and cell migration.
Figures


Similar articles
-
Proline-serine-threonine phosphatase interacting protein 1 (PSTPIP1) controls immune synapse stability in human T cells.J Allergy Clin Immunol. 2018 Dec;142(6):1947-1955. doi: 10.1016/j.jaci.2018.01.030. Epub 2018 Feb 9. J Allergy Clin Immunol. 2018. PMID: 29432774
-
Pombe Cdc15 homology (PCH) proteins: coordinators of membrane-cytoskeletal interactions.Trends Cell Biol. 2007 Mar;17(3):145-56. doi: 10.1016/j.tcb.2007.01.003. Epub 2007 Feb 12. Trends Cell Biol. 2007. PMID: 17296299 Review.
-
Pyrin-PSTPIP1 colocalises at the leading edge during cell migration.Cell Biol Int. 2015 Dec;39(12):1384-94. doi: 10.1002/cbin.10514. Epub 2015 Aug 14. Cell Biol Int. 2015. PMID: 26179737
-
Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis.J Cell Biol. 2006 Jan 16;172(2):269-79. doi: 10.1083/jcb.200508091. J Cell Biol. 2006. PMID: 16418535 Free PMC article.
-
The syndapin protein family: linking membrane trafficking with the cytoskeleton.J Cell Sci. 2004 Jul 1;117(Pt 15):3077-86. doi: 10.1242/jcs.01290. J Cell Sci. 2004. PMID: 15226389 Review.
Cited by
-
Leading from the Back: The Role of the Uropod in Neutrophil Polarization and Migration.Dev Cell. 2016 Jul 25;38(2):161-9. doi: 10.1016/j.devcel.2016.06.031. Dev Cell. 2016. PMID: 27459068 Free PMC article. Review.
-
The tandem PH domain-containing protein 2 (TAPP2) regulates chemokine-induced cytoskeletal reorganization and malignant B cell migration.PLoS One. 2013;8(2):e57809. doi: 10.1371/journal.pone.0057809. Epub 2013 Feb 27. PLoS One. 2013. PMID: 23460911 Free PMC article.
-
Linking up at the BAR: Oligomerization and F-BAR protein function.Cell Cycle. 2016 Aug 2;15(15):1977-85. doi: 10.1080/15384101.2016.1190893. Epub 2016 May 31. Cell Cycle. 2016. PMID: 27245932 Free PMC article. Review.
-
Contact-dependent T cell activation and T cell stopping require talin1.J Immunol. 2011 Dec 15;187(12):6256-67. doi: 10.4049/jimmunol.1102028. Epub 2011 Nov 9. J Immunol. 2011. PMID: 22075696 Free PMC article.
-
Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod.PLoS One. 2009;4(4):e5403. doi: 10.1371/journal.pone.0005403. Epub 2009 Apr 30. PLoS One. 2009. PMID: 19404397 Free PMC article.
References
-
- Babior B. M., Matzner Y. The familial Mediterranean fever gene—cloned at last. N. Engl. J. Med. 1997;337:1548–1549. - PubMed
-
- Badour K., Zhang J., Shi F., McGavin M. K., Rampersad V., Hardy L. A., Field D., Siminovitch K. A. The Wiskott-Aldrich syndrome protein acts downstream of CD2 and the CD2AP and PSTPIP1 adaptors to promote formation of the immunological synapse. Immunity. 2003;18:141–154. - PubMed
-
- Bairstow S. F., Ling K., Su X., Firestone A. J., Carbonara C., Anderson R. A. Type Igamma661 phosphatidylinositol phosphate kinase directly interacts with AP2 and regulates endocytosis. J. Biol. Chem. 2006;281:20632–20642. - PubMed
-
- Bennin D. A., Don A. S., Brake T., McKenzie J. L., Rosenbaum H., Ortiz L., DePaoli-Roach A. A., Horne M. C. Cyclin G2 associates with protein phosphatase 2A catalytic and regulatory B′ subunits in active complexes and induces nuclear aberrations and a G1/S phase cell cycle arrest. J. Biol. Chem. 2002;277:27449–27467. - PubMed
-
- Bretscher M. S. Moving membrane up to the front of migrating cells. Cell. 1996;85:465–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

