Synthesis of (+)-cortistatin A
- PMID: 18479104
- PMCID: PMC2652360
- DOI: 10.1021/ja8023466
Synthesis of (+)-cortistatin A
Abstract
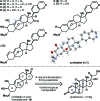
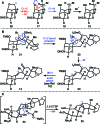
Cortistatin A is a marine steroid with highly selective and perhaps mechanistically unique antiangiogenic activity. Herein we report a synthesis of this natural product by way of "cortistatinone", an intermediate ideally suited for investigating the key pharmacophore of the cortistatin family. The synthesis begins with a terrestrial steroid and traverses a route to cortistatin A through the discovery of unique chemical reactivity. Specifically, we demonstrate the first example of a directed, geminal C-H bisoxidation, a new fragmentation cascade to access expanded B-ring steroid systems, a chemoselective cyclization to install the hallmark oxabicycle of the cortistatin family, and a remarkably selective hydrogenation reaction, which should find extensive use in future syntheses of the cortistatins and designed analogues. The synthesis displays a level of brevity, efficiency, and practicality that will be crucial in evaluating the medicinal potential of this fascinating class of marine steroids.
Figures

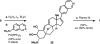
Similar articles
-
Stereodivergent synthesis of 17-alpha and 17-beta-alpharyl steroids: application and biological evaluation of D-ring cortistatin analogues.Angew Chem Int Ed Engl. 2009;48(24):4328-31. doi: 10.1002/anie.200901116. Angew Chem Int Ed Engl. 2009. PMID: 19434636 Free PMC article.
-
Total synthesis of cortistatins A and J.J Org Chem. 2011 Apr 15;76(8):2408-25. doi: 10.1021/jo2002616. Epub 2011 Mar 15. J Org Chem. 2011. PMID: 21405132
-
Stereoselective synthesis of core structure of cortistatin A.Org Lett. 2011 Jul 1;13(13):3514-7. doi: 10.1021/ol201327u. Epub 2011 Jun 9. Org Lett. 2011. PMID: 21651309
-
Chemistry of the cortistatins--a novel class of anti-angiogenic agents.Org Biomol Chem. 2010 Jun 28;8(13):2900-11. doi: 10.1039/c003935g. Epub 2010 May 12. Org Biomol Chem. 2010. PMID: 20463995 Review.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
Cited by
-
Synthesis of cortistatins A, J, K and L.Nat Chem. 2010 Oct;2(10):886-92. doi: 10.1038/nchem.794. Epub 2010 Aug 1. Nat Chem. 2010. PMID: 20861906 Free PMC article.
-
Anti-viral triterpenes: a review.Phytochem Rev. 2022;21(6):1761-1842. doi: 10.1007/s11101-022-09808-1. Epub 2022 Mar 5. Phytochem Rev. 2022. PMID: 35283698 Free PMC article. Review.
-
Total synthesis of (±)-cortistatin J from furan.J Am Chem Soc. 2011 Aug 17;133(32):12451-3. doi: 10.1021/ja206138d. Epub 2011 Jul 21. J Am Chem Soc. 2011. PMID: 21761900 Free PMC article.
-
Synthesis of the 8,19-Epoxysteroid Eurysterol A.Chemistry. 2020 Apr 1;26(19):4256-4260. doi: 10.1002/chem.202000585. Epub 2020 Mar 9. Chemistry. 2020. PMID: 32031278 Free PMC article.
-
Synthesis of angularly substituted trans-fused hydroindanes by convergent coupling of acyclic precursors.J Am Chem Soc. 2014 Jun 11;136(23):8209-12. doi: 10.1021/ja504374j. Epub 2014 May 29. J Am Chem Soc. 2014. PMID: 24856045 Free PMC article.
References
-
- Djerassi C.Steroids Made it Possible. In Profiles, Pathways and Dreams; Seeman J. I., Ed.; American Chemical Society: Washington, D.C., 1990.
-
- Aoki S.; Watanabe Y.; Sanagawa M.; Setiawan A.; Kotoku N.; Kobayashi M. J. Am. Chem. Soc. 2006, 128, 3148–3149. - PubMed
- Watanabe Y.; Aoki S.; Tanabe D.; Setiawan A.; Kobayashi M. Tetrahedron 2007, 63, 4074–4079.
- Aoki S.; Watanabe Y.; Tanabe D.; Setiawan A.; Arai M.; Kobayashi M. Tetrahedron Lett. 2007, 48, 4485–4488.
- Aoki S.; Watanabe Y.; Tanabe D.; Arai M.; Suna H.; Miyamoto K.; Tsujibo H.; Tsujikawa K.; Yamamoto H.; Kobayashi M. Bioorg. Med. Chem. 2007, 15, 6758–6762. - PubMed
-
- Folkman J. Nat. Rev. Drug Discovery 2007, 6, 273–286. - PubMed
-
-
Notably, the natural products themselves are so scarce that no authentic sample of cortistatin A could be spared for comparison to synthetic material, without impeding the biological studies already underway; personal communication with M. Kobayashi
-
-
- Bovicelli P.; Lupattelli P.; Mincione E.; Prencipe T.; Curci R. J. Org. Chem. 1992, 57, 2182–2184.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

