Neuregulin 1 in neural development, synaptic plasticity and schizophrenia
- PMID: 18478032
- PMCID: PMC2682371
- DOI: 10.1038/nrn2392
Neuregulin 1 in neural development, synaptic plasticity and schizophrenia
Abstract
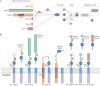
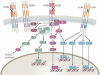
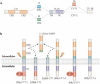
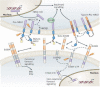
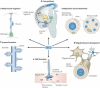
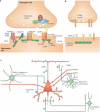
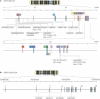
Schizophrenia is a highly debilitating mental disorder that affects approximately 1% of the general population, yet it continues to be poorly understood. Recent studies have identified variations in several genes that are associated with this disorder in diverse populations, including those that encode neuregulin 1 (NRG1) and its receptor ErbB4. The past few years have witnessed exciting progress in our knowledge of NRG1 and ErbB4 functions and the biological basis of the increased risk for schizophrenia that is potentially conferred by polymorphisms in the two genes. An improved understanding of the mechanisms by which altered function of NRG1 and ErbB4 contributes to schizophrenia might eventually lead to the development of more effective therapeutics.
Figures
Similar articles
-
Neuregulin-1 signalling and antipsychotic treatment: potential therapeutic targets in a schizophrenia candidate signalling pathway.Psychopharmacology (Berl). 2013 Mar;226(2):201-15. doi: 10.1007/s00213-013-3003-2. Epub 2013 Feb 7. Psychopharmacology (Berl). 2013. PMID: 23389757 Review.
-
ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation.Proc Natl Acad Sci U S A. 2010 Dec 14;107(50):21818-23. doi: 10.1073/pnas.1010669107. Epub 2010 Nov 24. Proc Natl Acad Sci U S A. 2010. PMID: 21106764 Free PMC article.
-
Neuregulin 1-erbB4 pathway in schizophrenia: From genes to an interactome.Brain Res Bull. 2010 Sep 30;83(3-4):132-9. doi: 10.1016/j.brainresbull.2010.04.011. Epub 2010 Apr 28. Brain Res Bull. 2010. PMID: 20433909 Free PMC article. Review.
-
Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling.Nature. 2010 Apr 29;464(7293):1376-80. doi: 10.1038/nature08928. Epub 2010 Apr 14. Nature. 2010. PMID: 20393464
-
The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity.Neuron. 2007 May 24;54(4):583-97. doi: 10.1016/j.neuron.2007.03.028. Neuron. 2007. PMID: 17521571 Free PMC article.
Cited by
-
Shank1 regulates excitatory synaptic transmission in mouse hippocampal parvalbumin-expressing inhibitory interneurons.Eur J Neurosci. 2015 Apr;41(8):1025-35. doi: 10.1111/ejn.12877. Epub 2015 Mar 25. Eur J Neurosci. 2015. PMID: 25816842 Free PMC article.
-
CSF levels of the BACE1 substrate NRG1 correlate with cognition in Alzheimer's disease.Alzheimers Res Ther. 2020 Jul 20;12(1):88. doi: 10.1186/s13195-020-00655-w. Alzheimers Res Ther. 2020. PMID: 32690068 Free PMC article.
-
Identification of visual cortex cell types and species differences using single-cell RNA sequencing.Nat Commun. 2022 Nov 12;13(1):6902. doi: 10.1038/s41467-022-34590-1. Nat Commun. 2022. PMID: 36371428 Free PMC article.
-
Aberrant neuregulin 1 signaling in amyotrophic lateral sclerosis.J Neuropathol Exp Neurol. 2012 Feb;71(2):104-15. doi: 10.1097/NEN.0b013e3182423c43. J Neuropathol Exp Neurol. 2012. PMID: 22249457 Free PMC article.
-
Huntington's disease and its therapeutic target genes: a global functional profile based on the HD Research Crossroads database.BMC Neurol. 2012 Jun 28;12:47. doi: 10.1186/1471-2377-12-47. BMC Neurol. 2012. PMID: 22741533 Free PMC article.
References
-
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. 1963;20:140–144. - PubMed
-
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J. Psychopharmacol. 1999;13:358–371. - PubMed
-
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. - PubMed
-
- Tan W, et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 2007;282:24343–24351. - PubMed
-
- Falls DL. Neuregulins: functions, forms, and signalling strategies. Exp. Cell Res. 2003;284:14–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

