TRAIL recombinant adenovirus triggers robust apoptosis in multidrug-resistant HL-60/Vinc cells preferentially through death receptor DR5
- PMID: 18476767
- PMCID: PMC2733364
- DOI: 10.1089/hum.2008.001
TRAIL recombinant adenovirus triggers robust apoptosis in multidrug-resistant HL-60/Vinc cells preferentially through death receptor DR5
Abstract
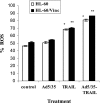
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising cancer therapeutic because of its highly selective apoptosis-inducing action on neoplastic versus normal cells. However, some cancer cells express resistance to recombinant soluble TRAIL. To overcome this problem, we used a TRAIL adenovirus (Ad5/35-TRAIL) to induce apoptosis in a drug-sensitive and multidrug-resistant variant of HL-60 leukemia cells and determined the molecular mechanisms of Ad5/35-TRAIL-induced apoptosis. Ad5/35-TRAIL did not induce apoptosis in normal human lymphocytes, but caused massive apoptosis in acute myelocytic leukemia cells. It triggered more efficient apoptosis in drug-resistant HL-60/Vinc cells than in HL-60 cells. Treating the cells with anti-DR4 and anti-DR5 neutralizing antibodies (particularly anti-DR5) reduced, whereas anti-DcR1 antibody enhanced, the apoptosis triggered by Ad5/35-TRAIL. Whereas Ad5/35-TRAIL induced apoptosis in both cell lines through activation of caspase-3 and caspase-10, known to link the cell death receptor pathway to the mitochondrial pathway, it triggered increased mitochondrial membrane potential change (m) only in HL-60/Vinc cells. Ad5/35-TRAIL also increased the production of reactive oxygen species, which play an important role in apoptosis. Therefore, using Ad5/35-TRAIL may be an effective therapeutic strategy for eliminating TRAIL-resistant malignant cells and these studies may provide clues to treat and eradicate acute myelocytic leukemias.
Figures
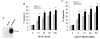
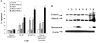
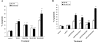


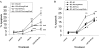

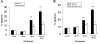
Similar articles
-
P-glycoprotein enhances TRAIL-triggered apoptosis in multidrug resistant cancer cells by interacting with the death receptor DR5.Biochem Pharmacol. 2006 Jul 28;72(3):293-307. doi: 10.1016/j.bcp.2006.04.024. Epub 2006 May 4. Biochem Pharmacol. 2006. PMID: 16753135
-
Expression of TRAIL (Apo2L), DR4 (TRAIL receptor 1), DR5 (TRAIL receptor 2) and TRID (TRAIL receptor 3) genes in multidrug resistant human acute myeloid leukemia cell lines that overexpress MDR 1 (HL60/Tax) or MRP (HL60/AR).Int J Oncol. 2000 Jun;16(6):1137-9. doi: 10.3892/ijo.16.6.1137. Int J Oncol. 2000. PMID: 10811986
-
Ewing's sarcoma family tumors are sensitive to tumor necrosis factor-related apoptosis-inducing ligand and express death receptor 4 and death receptor 5.Cancer Res. 2001 Mar 15;61(6):2704-12. Cancer Res. 2001. PMID: 11289151
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Selective TRAIL-triggered apoptosis due to overexpression of TRAIL death receptor 5 (DR5) in P-glycoprotein-bearing multidrug resistant CEM/VBL1000 human leukemia cells.Int J Biochem Mol Biol. 2010;1(1):90-100. Epub 2010 Jul 18. Int J Biochem Mol Biol. 2010. PMID: 20953314 Free PMC article.
-
Targeting the Anti-Apoptotic Protein c-FLIP for Cancer Therapy.Cancers (Basel). 2011 Jun;3(2):1639-71. doi: 10.3390/cancers3021639. Cancers (Basel). 2011. PMID: 22348197 Free PMC article.
-
c-FLIP knockdown induces ligand-independent DR5-, FADD-, caspase-8-, and caspase-9-dependent apoptosis in breast cancer cells.Biochem Pharmacol. 2008 Dec 15;76(12):1694-704. doi: 10.1016/j.bcp.2008.09.007. Epub 2008 Sep 17. Biochem Pharmacol. 2008. PMID: 18840411 Free PMC article.
-
The extracellular matrix glycoprotein elastin microfibril interface located protein 2: a dual role in the tumor microenvironment.Neoplasia. 2010 Apr;12(4):294-304. doi: 10.1593/neo.91930. Neoplasia. 2010. PMID: 20360940 Free PMC article.
References
-
- Abedini M.R. Qiu Q. Yan X. Tsang B.K. Possible role of FLICE-like inhibitory protein (FLIP) in chemoresistant ovarian cancer cells in vitro. Oncogene. 2004;23:6997–7004. - PubMed
-
- Ashkenazi A. Pai R.C. Fong S. Leung S. Lawrence D.A. Marsters S.A. Blackie C. Chang L. McMurtrey A.E. Hebert A. Deforge L. Koumenis I.L. Lewis D. Harris L. Bussiere J. Koeppen H. Shahrokh Z. Schwall R.H. Safety and antitumor activity of recombinant soluble Apo2 ligand. J. Clin. Invest. 1999;104:155–162. - PMC - PubMed
-
- Balamotis M.A. Huang K. Mitani K. Efficient delivery and stable gene expression in a hematopoietic cell line using a chimeric serotype 35 fiber pseudotyped helper-dependent adenoviral vector. Virology. 2004;324:229–237. - PubMed
-
- Bedi A. Zehnbauer B.A. Barber J.P. Sharkis S.J. Jones R.J. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994;83:2038–2044. - PubMed
-
- Bedi A. Barber J.P. Bedi G.C. El-Deiry W.S. Sidransky D. Vala M.S. Akhtar A.J. Hilton J. Jones R.J. BCR-ABL mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: A mechanism of resistance to multiple anticancer agents. Blood. 1995;86:1148–1158. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

