Retromer
- PMID: 18472259
- PMCID: PMC2833274
- DOI: 10.1016/j.ceb.2008.03.009
Retromer
Abstract
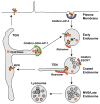
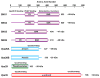
The retromer is a heteropentameric complex that associates with the cytosolic face of endosomes and mediates retrograde transport of transmembrane cargo from endosomes to the trans-Golgi network. The mammalian retromer complex comprises a sorting nexin dimer composed of a still undefined combination of SNX1, SNX2, SNX5 and SNX6, and a cargo-recognition trimer composed of Vps26, Vps29 and Vps35. The SNX subunits contain PX and BAR domains that allow binding to PI(3)P enriched, highly curved membranes of endosomal vesicles and tubules, while Vps26, Vps29 and Vps35 have arrestin, phosphoesterase and alpha-solenoid folds, respectively. Recent studies have implicated retromer in a broad range of physiological, developmental and pathological processes, underscoring the critical nature of retrograde transport mediated by this complex.
Figures
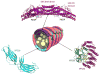

Similar articles
-
Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport.J Cell Biol. 2017 Nov 6;216(11):3677-3693. doi: 10.1083/jcb.201702137. Epub 2017 Sep 21. J Cell Biol. 2017. PMID: 28935632 Free PMC article.
-
Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors.Mol Cell Biol. 2007 Feb;27(3):1112-24. doi: 10.1128/MCB.00156-06. Epub 2006 Nov 13. Mol Cell Biol. 2007. PMID: 17101778 Free PMC article.
-
A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer.J Cell Sci. 2007 Jan 1;120(Pt 1):45-54. doi: 10.1242/jcs.03302. Epub 2006 Dec 5. J Cell Sci. 2007. PMID: 17148574
-
Updated Insight into the Physiological and Pathological Roles of the Retromer Complex.Int J Mol Sci. 2017 Jul 25;18(8):1601. doi: 10.3390/ijms18081601. Int J Mol Sci. 2017. PMID: 28757549 Free PMC article. Review.
-
Retromer and the cation-independent mannose 6-phosphate receptor-Time for a trial separation?Traffic. 2018 Feb;19(2):150-152. doi: 10.1111/tra.12542. Epub 2017 Dec 21. Traffic. 2018. PMID: 29135085 Free PMC article. Review.
Cited by
-
Vps35 loss promotes hyperresorptive osteoclastogenesis and osteoporosis via sustained RANKL signaling.J Cell Biol. 2013 Mar 18;200(6):821-37. doi: 10.1083/jcb.201207154. J Cell Biol. 2013. PMID: 23509071 Free PMC article.
-
SorLA is a molecular link for retromer-dependent sorting of the Amyloid precursor protein.Commun Integr Biol. 2012 Nov 1;5(6):616-9. doi: 10.4161/cib.21433. Commun Integr Biol. 2012. PMID: 23740096 Free PMC article.
-
VPS35 regulates cell surface recycling and signaling of dopamine receptor D1.Neurobiol Aging. 2016 Oct;46:22-31. doi: 10.1016/j.neurobiolaging.2016.05.016. Epub 2016 May 21. Neurobiol Aging. 2016. PMID: 27460146 Free PMC article.
-
The lipid kinase PI4KIIIβ preserves lysosomal identity.EMBO J. 2013 Feb 6;32(3):324-39. doi: 10.1038/emboj.2012.341. Epub 2012 Dec 21. EMBO J. 2013. PMID: 23258225 Free PMC article.
-
VPS35 regulates developing mouse hippocampal neuronal morphogenesis by promoting retrograde trafficking of BACE1.Biol Open. 2012 Dec 15;1(12):1248-57. doi: 10.1242/bio.20122451. Epub 2012 Oct 11. Biol Open. 2012. PMID: 23259059 Free PMC article.
References
-
- Seaman MN. Recycle your receptors with retromer. Trends Cell Biol. 2005;15:68–75. - PubMed
-
- Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. - PubMed
-
- Verges M. Retromer and sorting nexins in development. Front Biosci. 2007;12:3825–3851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

