Regulation of the sumoylation system in gene expression
- PMID: 18468876
- PMCID: PMC2495007
- DOI: 10.1016/j.ceb.2008.03.014
Regulation of the sumoylation system in gene expression
Abstract
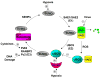
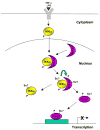
Protein sumoylation has emerged as an important regulatory mechanism for the transcriptional machinery. Sumoylation is a highly dynamic process that is regulated in response to cellular stimuli or pathogenic challenges. Altered activity of the small ubiquitin-like modifier (SUMO) conjugation system is associated with human cancers and inflammation. Thus, understanding the regulation of protein sumoylation is important for the design of SUMO-based therapeutic strategies for the treatment of human diseases. Recent studies indicate that the sumoylation system can be regulated through multiple mechanisms, including the regulation of the expression of various components of the sumoylation pathway, and the modulation of the activity of SUMO enzymes. In addition, extracellular stimuli can signal the nucleus to trigger the rapid promoter recruitment of SUMO E3 ligases, resulting in the immediate repression of transcription. Finally, the sumoylation system can also be regulated through crosstalk with other post-translational modifications, including phosphorylation, ubiquitination, and acetylation.
Figures
Similar articles
-
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a SUMO E3 ligase that is SIM-dependent and SUMO-2/3-specific.J Biol Chem. 2010 Feb 19;285(8):5266-73. doi: 10.1074/jbc.M109.088088. Epub 2009 Dec 24. J Biol Chem. 2010. PMID: 20034935 Free PMC article.
-
Identification of a new small ubiquitin-like modifier (SUMO)-interacting motif in the E3 ligase PIASy.J Biol Chem. 2017 Jun 16;292(24):10230-10238. doi: 10.1074/jbc.M117.789982. Epub 2017 Apr 28. J Biol Chem. 2017. PMID: 28455449 Free PMC article.
-
Roles of Ubiquitination and SUMOylation in DNA Damage Response.Curr Issues Mol Biol. 2020;35:59-84. doi: 10.21775/cimb.035.059. Epub 2019 Aug 18. Curr Issues Mol Biol. 2020. PMID: 31422933 Review.
-
Mechanisms, regulation and consequences of protein SUMOylation.Biochem J. 2010 May 13;428(2):133-45. doi: 10.1042/BJ20100158. Biochem J. 2010. PMID: 20462400 Free PMC article. Review.
-
SUMO: getting it on.Biochem Soc Trans. 2007 Dec;35(Pt 6):1409-13. doi: 10.1042/BST0351409. Biochem Soc Trans. 2007. PMID: 18031233 Review.
Cited by
-
SUMO modification of NZFP mediates transcriptional repression through TBP binding.Mol Cells. 2013 Jan;35(1):70-8. doi: 10.1007/s10059-013-2281-1. Epub 2012 Dec 21. Mol Cells. 2013. PMID: 23269432 Free PMC article.
-
Regulation of IkappaBalpha function and NF-kappaB signaling: AEBP1 is a novel proinflammatory mediator in macrophages.Mediators Inflamm. 2010;2010:823821. doi: 10.1155/2010/823821. Epub 2010 Apr 12. Mediators Inflamm. 2010. PMID: 20396415 Free PMC article. Review.
-
SENP1 participates in the dynamic regulation of Elk-1 SUMOylation.Biochem J. 2010 May 13;428(2):247-54. doi: 10.1042/BJ20091948. Biochem J. 2010. PMID: 20337593 Free PMC article.
-
Epigenetic dysfunctional diseases and therapy for infection and inflammation.Biochim Biophys Acta Mol Basis Dis. 2017 Feb;1863(2):518-528. doi: 10.1016/j.bbadis.2016.11.030. Epub 2016 Dec 3. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 27919711 Free PMC article. Review.
-
PPARgamma1 and LXRalpha face a new regulator of macrophage cholesterol homeostasis and inflammatory responsiveness, AEBP1.Nucl Recept Signal. 2010 Apr 16;8:e004. doi: 10.1621/nrs.08004. Nucl Recept Signal. 2010. PMID: 20419060 Free PMC article. Review.
References
-
- Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. - PubMed
-
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. - PubMed
-
- Melchior F. SUMO--nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. - PubMed
-
- Shuai K, Liu B. Regulation of gene-activation pathways by PIAS proteins in the immune system. Nat Rev Immunol. 2005;5:593–605. - PubMed
-
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

