Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria
- PMID: 18466296
- PMCID: PMC2615189
- DOI: 10.1111/j.1365-2958.2008.06274.x
Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria
Abstract
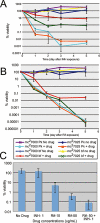
Successful treatment of human tuberculosis requires 6-9 months' therapy with multiple antibiotics. Incomplete clearance of tubercle bacilli frequently results in disease relapse, presumably as a result of reactivation of persistent drug-tolerant Mycobacterium tuberculosis cells, although the nature and location of these persisters are not known. In other pathogens, antibiotic tolerance is often associated with the formation of biofilms--organized communities of surface-attached cells--but physiologically and genetically defined M. tuberculosis biofilms have not been described. Here, we show that M. tuberculosis forms biofilms with specific environmental and genetic requirements distinct from those for planktonic growth, which contain an extracellular matrix rich in free mycolic acids, and harbour an important drug-tolerant population that persist despite exposure to high levels of antibiotics.
Figures



Similar articles
-
Keto-mycolic acid-dependent pellicle formation confers tolerance to drug-sensitive Mycobacterium tuberculosis.mBio. 2013 May 7;4(3):e00222-13. doi: 10.1128/mBio.00222-13. mBio. 2013. PMID: 23653446 Free PMC article.
-
Mycobacterial Biofilms: Revisiting Tuberculosis Bacilli in Extracellular Necrotizing Lesions.Microbiol Spectr. 2017 Jun;5(3):10.1128/microbiolspec.tbtb2-0024-2016. doi: 10.1128/microbiolspec.TBTB2-0024-2016. Microbiol Spectr. 2017. PMID: 28597824 Free PMC article. Review.
-
Adaptation of Mycobacterium tuberculosis to Biofilm Growth Is Genetically Linked to Drug Tolerance.Antimicrob Agents Chemother. 2019 Oct 22;63(11):e01213-19. doi: 10.1128/AAC.01213-19. Print 2019 Nov. Antimicrob Agents Chemother. 2019. PMID: 31501144 Free PMC article.
-
Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms.Expert Rev Anti Infect Ther. 2012 Sep;10(9):1055-66. doi: 10.1586/eri.12.88. Expert Rev Anti Infect Ther. 2012. PMID: 23106280 Free PMC article. Review.
-
Mycobacterium Tuberculosis Biofilm - A new perspective.Indian J Tuberc. 2015 Jan;62(1):4-6. doi: 10.1016/j.ijtb.2015.02.028. Epub 2015 Mar 20. Indian J Tuberc. 2015. PMID: 25857559 No abstract available.
Cited by
-
The role of chemoenzymatic synthesis in advancing trehalose analogues as tools for combatting bacterial pathogens.Chem Commun (Camb). 2020 Oct 1;56(78):11528-11547. doi: 10.1039/d0cc04955g. Chem Commun (Camb). 2020. PMID: 32914793 Free PMC article.
-
Mycolic acids: deciphering and targeting the Achilles' heel of the tubercle bacillus.Mol Microbiol. 2015 Oct;98(1):7-16. doi: 10.1111/mmi.13101. Epub 2015 Jul 30. Mol Microbiol. 2015. PMID: 26135034 Free PMC article. Review.
-
Exposure to a cutinase-like serine esterase triggers rapid lysis of multiple mycobacterial species.J Biol Chem. 2013 Jan 4;288(1):382-92. doi: 10.1074/jbc.M112.419754. Epub 2012 Nov 15. J Biol Chem. 2013. PMID: 23155047 Free PMC article.
-
Functional Amyloid and Other Protein Fibers in the Biofilm Matrix.J Mol Biol. 2018 Oct 12;430(20):3642-3656. doi: 10.1016/j.jmb.2018.07.026. Epub 2018 Aug 8. J Mol Biol. 2018. PMID: 30098341 Free PMC article. Review.
-
Mycobacterium abscessus Cells Have Altered Antibiotic Tolerance and Surface Glycolipids in Artificial Cystic Fibrosis Sputum Medium.Antimicrob Agents Chemother. 2019 Jun 24;63(7):e02488-18. doi: 10.1128/AAC.02488-18. Print 2019 Jul. Antimicrob Agents Chemother. 2019. PMID: 31010859 Free PMC article.
References
-
- Anderson GG, Dodson KW, Hooton TM, Hultgren SJ. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 2004;12:424–430. - PubMed
-
- Balaban NQ, Merrin J, Chait R, Kowalik L, Leibler S. Bacterial persistence as a phenotypic switch. Science. 2004;305:1622–1625. - PubMed
-
- Bardarov S, Pavelka MS, Sambandamurthy V, Larsen M, Tufariello J, Chan J, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148:3007–3017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

