Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells
- PMID: 18451337
- PMCID: PMC2737681
- DOI: 10.1161/CIRCRESAHA.107.167080
Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell-cell adhesions in endothelial cells
Erratum in
- Circ Res. 2013 Oct 25;113(10):e101
Abstract
Vascular endothelial growth factor (VEGF) binding induces phosphorylation of VEGF receptor (VEGFR)2 in tyrosine, which is followed by disruption of VE-cadherin-mediated cell-cell contacts of endothelial cells (ECs), thereby stimulating EC proliferation and migration to promote angiogenesis. Tyrosine phosphorylation events are controlled by the balance of activation of protein tyrosine kinases and protein tyrosine phosphatases (PTPs). Little is known about the role of endogenous PTPs in VEGF signaling in ECs. In this study, we found that PTP1B expression and activity are markedly increased in mice hindlimb ischemia model of angiogenesis. In ECs, overexpression of PTP1B, but not catalytically inactive mutant PTP1B-C/S, inhibits VEGF-induced phosphorylation of VEGFR2 and extracellular signal-regulated kinase 1/2, as well as EC proliferation, whereas knockdown of PTP1B by small interfering RNA enhances these responses, suggesting that PTP1B negatively regulates VEGFR2 signaling in ECs. VEGF-induced p38 mitogen-activated protein kinase phosphorylation and EC migration are not affected by PTP1B overexpression or knockdown. In vivo dephosphorylation and cotransfection assays reveal that PTP1B binds to VEGFR2 cytoplasmic domain in vivo and directly dephosphorylates activated VEGFR2 immunoprecipitates from human umbilical vein endothelial cells. Overexpression of PTP1B stabilizes VE-cadherin-mediated cell-cell adhesions by reducing VE-cadherin tyrosine phosphorylation, whereas PTP1B small interfering RNA causes opposite effects with increasing endothelial permeability, as measured by transendothelial electric resistance. In summary, PTP1B negatively regulates VEGFR2 receptor activation via binding to the VEGFR2, as well as stabilizes cell-cell adhesions through reducing tyrosine phosphorylation of VE-cadherin. Induction of PTP1B by hindlimb ischemia may represent an important counterregulatory mechanism that blunts overactivation of VEGFR2 during angiogenesis in vivo.
Figures



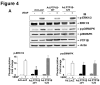

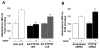
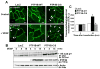


Similar articles
-
PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells.Circulation. 2014 Sep 9;130(11):902-9. doi: 10.1161/CIRCULATIONAHA.114.009683. Epub 2014 Jun 30. Circulation. 2014. PMID: 24982127 Free PMC article.
-
IQGAP1 mediates VE-cadherin-based cell-cell contacts and VEGF signaling at adherence junctions linked to angiogenesis.Arterioscler Thromb Vasc Biol. 2006 Sep;26(9):1991-7. doi: 10.1161/01.ATV.0000231524.14873.e7. Epub 2006 Jun 8. Arterioscler Thromb Vasc Biol. 2006. PMID: 16763158
-
Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis.Circ Res. 2005 Mar 4;96(4):467-75. doi: 10.1161/01.RES.0000158286.51045.16. Epub 2005 Feb 3. Circ Res. 2005. PMID: 15692085
-
Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family.Cardiovasc Res. 2001 Feb 16;49(3):568-81. doi: 10.1016/s0008-6363(00)00268-6. Cardiovasc Res. 2001. PMID: 11166270 Review.
-
Human Protein Tyrosine Phosphatase 1B (PTP1B): From Structure to Clinical Inhibitor Perspectives.Int J Mol Sci. 2022 Jun 24;23(13):7027. doi: 10.3390/ijms23137027. Int J Mol Sci. 2022. PMID: 35806030 Free PMC article. Review.
Cited by
-
Protein tyrosine phosphatases in cell adhesion.Biochem J. 2021 Mar 12;478(5):1061-1083. doi: 10.1042/BCJ20200511. Biochem J. 2021. PMID: 33710332 Free PMC article. Review.
-
Topical vanadate improves tensile strength and alters collagen organisation of excisional wounds in a mouse model.Wound Repair Regen. 2023 Jan;31(1):77-86. doi: 10.1111/wrr.13062. Epub 2022 Dec 14. Wound Repair Regen. 2023. PMID: 36484112 Free PMC article.
-
An integrated approach for experimental target identification of hypoxia-induced miR-210.J Biol Chem. 2009 Dec 11;284(50):35134-43. doi: 10.1074/jbc.M109.052779. Epub 2009 Oct 13. J Biol Chem. 2009. PMID: 19826008 Free PMC article.
-
Sec14l3 potentiates VEGFR2 signaling to regulate zebrafish vasculogenesis.Nat Commun. 2019 Apr 8;10(1):1606. doi: 10.1038/s41467-019-09604-0. Nat Commun. 2019. PMID: 30962435 Free PMC article.
-
Proteomic Maps of Human Gastrointestinal Stromal Tumor Subgroups.Mol Cell Proteomics. 2019 May;18(5):923-935. doi: 10.1074/mcp.RA119.001361. Epub 2019 Feb 25. Mol Cell Proteomics. 2019. PMID: 30804049 Free PMC article.
References
-
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001:RE21. - PubMed
-
- Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23:434–445. - PubMed
-
- Wallez Y, Vilgrain I, Huber P. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med. 2006;16:55–59. - PubMed
-
- Sugano M, Tsuchida K, Makino N. A protein tyrosine phosphatase inhibitor accelerates angiogenesis in a rat model of hindlimb ischemia. J Cardiovasc Pharmacol. 2004;44:460–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

