Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism
- PMID: 18451260
- PMCID: PMC2528849
- DOI: 10.1210/er.2007-0025
Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism
Abstract
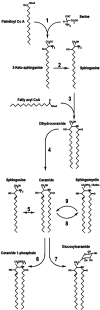
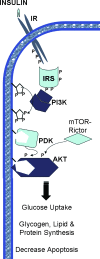
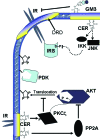
Obesity and dyslipidemia are risk factors for metabolic disorders including diabetes and cardiovascular disease. Sphingolipids such as ceramide and glucosylceramides, while being a relatively minor component of the lipid milieu in most tissues, may be among the most pathogenic lipids in the onset of the sequelae associated with excess adiposity. Circulating factors associated with obesity (e.g., saturated fatty acids, inflammatory cytokines) selectively induce enzymes that promote sphingolipid synthesis, and lipidomic profiling reveals relationships between tissue sphingolipid levels and certain metabolic diseases. Moreover, studies in cultured cells and isolated tissues implicate sphingolipids in certain cellular events associated with diabetes and cardiovascular disease, including insulin resistance, pancreatic beta-cell failure, cardiomyopathy, and vascular dysfunction. However, definitive evidence that sphingolipids contribute to insulin resistance, diabetes, and atherosclerosis has come only recently, as researchers have found that pharmacological inhibition or genetic ablation of enzymes controlling sphingolipid synthesis in rodents ameliorates each of these conditions. Herein we will review the role of ceramide and other sphingolipid metabolites in insulin resistance, beta-cell failure, cardiomyopathy, and vascular dysfunction, focusing on these in vivo studies that identify enzymes controlling sphingolipid metabolism as therapeutic targets for combating metabolic disease.
Figures

Similar articles
-
Ceramides - Lipotoxic Inducers of Metabolic Disorders.Trends Endocrinol Metab. 2015 Oct;26(10):538-550. doi: 10.1016/j.tem.2015.07.006. Trends Endocrinol Metab. 2015. PMID: 26412155 Review.
-
Sphingolipids: players in the pathology of metabolic disease.Trends Endocrinol Metab. 2009 Jan;20(1):34-42. doi: 10.1016/j.tem.2008.09.004. Epub 2008 Nov 13. Trends Endocrinol Metab. 2009. PMID: 19008117 Review.
-
Sphingolipids and Lipoproteins in Health and Metabolic Disorders.Trends Endocrinol Metab. 2017 Jul;28(7):506-518. doi: 10.1016/j.tem.2017.03.005. Epub 2017 Apr 24. Trends Endocrinol Metab. 2017. PMID: 28462811 Free PMC article. Review.
-
Sphingolipids: agents provocateurs in the pathogenesis of insulin resistance.Diabetologia. 2011 Jul;54(7):1596-607. doi: 10.1007/s00125-011-2127-3. Epub 2011 Apr 6. Diabetologia. 2011. PMID: 21468641 Review.
-
Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance.Cell Metab. 2007 Mar;5(3):167-79. doi: 10.1016/j.cmet.2007.01.002. Cell Metab. 2007. PMID: 17339025
Cited by
-
Adiponectin Resistance in Obesity: Adiponectin Leptin/Insulin Interaction.Adv Exp Med Biol. 2024;1460:431-462. doi: 10.1007/978-3-031-63657-8_15. Adv Exp Med Biol. 2024. PMID: 39287861 Review.
-
Mendelian randomization analysis reveals causal effects of blood lipidome on gestational diabetes mellitus.Cardiovasc Diabetol. 2024 Sep 11;23(1):335. doi: 10.1186/s12933-024-02429-2. Cardiovasc Diabetol. 2024. PMID: 39261922 Free PMC article.
-
High-fat feeding drives the intestinal production and assembly of C16:0 ceramides in chylomicrons.Sci Adv. 2024 Aug 23;10(34):eadp2254. doi: 10.1126/sciadv.adp2254. Epub 2024 Aug 23. Sci Adv. 2024. PMID: 39178255 Free PMC article.
-
Application of sphingolipid-based nanocarriers in drug delivery: an overview.Ther Deliv. 2024;15(8):619-637. doi: 10.1080/20415990.2024.2377066. Epub 2024 Jul 29. Ther Deliv. 2024. PMID: 39072358 Review.
-
The Potential Mechanism of Remission in Type 2 Diabetes Mellitus After Vertical Sleeve Gastrectomy.Obes Surg. 2024 Aug;34(8):3071-3083. doi: 10.1007/s11695-024-07378-z. Epub 2024 Jul 1. Obes Surg. 2024. PMID: 38951388 Review.
References
-
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM 2006 Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295:1549–1555 - PubMed
-
- Shaw DI, Hall WL, Williams CM 2005 Metabolic syndrome: what is it and what are the implications? Proc Nutr Soc 64:349–357 - PubMed
-
- Popkin BM, Kim S, Rusev ER, Du S, Zizza C 2006 Measuring the full economic costs of diet, physical activity and obesity-related chronic diseases. Obes Rev 7:271–293 - PubMed
-
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS 2005 A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352:1138–1145 - PubMed
-
- Merrill Jr AH 2002 De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem 277:25843–25846 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

