Vaccine adjuvants alter TCR-based selection thresholds
- PMID: 18450485
- PMCID: PMC2695494
- DOI: 10.1016/j.immuni.2008.03.014
Vaccine adjuvants alter TCR-based selection thresholds
Abstract
How T cell receptor (TCR) specificity evolves in vivo after protein vaccination is central to the development of helper T (Th) cell function. Most models of clonal selection in the Th cell compartment favor TCR affinity-based thresholds. Here, we demonstrated that depot-forming vaccine adjuvants did not require Toll-like receptor (TLR) agonists to induce clonal dominance in antigen-specific Th cell responses. However, readily dispersible adjuvants using TLR-9 and TLR-4 agonists skewed TCR repertoire usage by increasing TCR selection thresholds and enhancing antigen-specific clonal expansion. In this manner, vaccine adjuvants control the local accumulation of Th cells expressing TCR with the highest peptide MHC class II binding. Clonal composition was altered by mechanisms that blocked the local propagation of clonotypes independently of antigen dose and not as a consequence of interclonal competition. This capacity of adjuvants to modify antigen-specific Th cell clonal composition has fundamental implications for the design of future protein subunit vaccines.
Figures
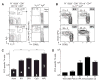

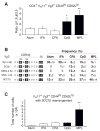
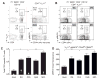
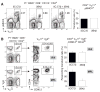
Comment in
-
Taking a toll road to better vaccines.Immunity. 2008 May;28(5):602-4. doi: 10.1016/j.immuni.2008.04.008. Immunity. 2008. PMID: 18482564
Similar articles
-
A TCR affinity threshold regulates memory CD4 T cell differentiation following vaccination.J Immunol. 2012 Sep 1;189(5):2309-17. doi: 10.4049/jimmunol.1200453. Epub 2012 Jul 27. J Immunol. 2012. PMID: 22844120 Free PMC article.
-
Regulation of T Helper Cell Fate by TCR Signal Strength.Front Immunol. 2020 May 19;11:624. doi: 10.3389/fimmu.2020.00624. eCollection 2020. Front Immunol. 2020. PMID: 32508803 Free PMC article. Review.
-
Taking a toll road to better vaccines.Immunity. 2008 May;28(5):602-4. doi: 10.1016/j.immuni.2008.04.008. Immunity. 2008. PMID: 18482564
-
Clonal selection of helper T cells is determined by an affinity threshold with no further skewing of TCR binding properties.Immunity. 2004 Nov;21(5):669-79. doi: 10.1016/j.immuni.2004.09.008. Immunity. 2004. PMID: 15539153
-
Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines?Front Immunol. 2018 Dec 13;9:2874. doi: 10.3389/fimmu.2018.02874. eCollection 2018. Front Immunol. 2018. PMID: 30619259 Free PMC article. Review.
Cited by
-
A TCR affinity threshold regulates memory CD4 T cell differentiation following vaccination.J Immunol. 2012 Sep 1;189(5):2309-17. doi: 10.4049/jimmunol.1200453. Epub 2012 Jul 27. J Immunol. 2012. PMID: 22844120 Free PMC article.
-
How do adjuvants enhance immune responses?Elife. 2024 Aug 13;13:e101259. doi: 10.7554/eLife.101259. Elife. 2024. PMID: 39136115 Free PMC article.
-
Regulation of T Helper Cell Fate by TCR Signal Strength.Front Immunol. 2020 May 19;11:624. doi: 10.3389/fimmu.2020.00624. eCollection 2020. Front Immunol. 2020. PMID: 32508803 Free PMC article. Review.
-
Stepwise B-cell-dependent expansion of T helper clonotypes diversifies the T-cell response.Nat Commun. 2016 Jan 5;7:10281. doi: 10.1038/ncomms10281. Nat Commun. 2016. PMID: 26728651 Free PMC article.
-
TCR Signaling Emerges from the Sum of Many Parts.Front Immunol. 2012 Jun 25;3:159. doi: 10.3389/fimmu.2012.00159. eCollection 2012. Front Immunol. 2012. PMID: 22737151 Free PMC article.
References
-
- Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–107. - PubMed
-
- Baldridge JR, McGowan P, Evans JT, Cluff C, Mossman S, Johnson D, Persing D. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin Biol Ther. 2004;4:1129–1138. - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
-
- Bikah G, Pogue-Caley RR, McHeyzer-Williams LJ, McHeyzer-Williams MG. Regulating T helper cell immunity through antigen responsiveness and calcium entry. Nat Immunol. 2000;1:402–412. - PubMed
-
- Billiau A, Matthys P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

