CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-kappaB activation
- PMID: 18450452
- PMCID: PMC2587165
- DOI: 10.1016/j.cub.2008.04.017
CARP-2 is an endosome-associated ubiquitin ligase for RIP and regulates TNF-induced NF-kappaB activation
Abstract
Background: The proinflammatory cytokine tumor necrosis factor-alpha (TNF-alpha) elicits cellular responses by signaling through a receptor complex that includes the essential adaptor molecule RIP. One important consequence of signaling is activation of the transcription factor NF-kappaB, and failure to downregulate TNF-induced NF-kappaB transcriptional activity results in chronic inflammation and death. Internalization of the receptor complex plays an important regulatory role in TNF signaling.
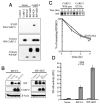
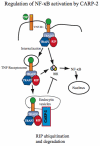
Results: We report that CARP-2, a RING domain-containing ubiquitin protein ligase (E3), is a negative regulator of TNF-induced NF-kappaB activation. By virtue of its phospholipid-binding FYVE domain, CARP-2 localized to endocytic vesicles, where it interacted with internalized TNF-receptor complex, resulting in RIP ubiquitination and degradation. Knockdown of CARP-2 stabilized TNFR1-associated polyubiquitinated RIP levels after TNF simulation and enhanced activation of NF-kappaB.
Conclusions: CARP-2 acts at the level of endocytic vesicles to limit the intensity of TNF-induced NF-kappaB activation by the regulated elimination of a necessary signaling component within the receptor complex.
Figures

Comment in
-
CARP2 deficiency does not alter induction of NF-kappaB by TNFalpha.Curr Biol. 2009 Jan 13;19(1):R15-7; author reply R17-9. doi: 10.1016/j.cub.2008.11.040. Curr Biol. 2009. PMID: 19138581 No abstract available.
Similar articles
-
De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling.Nature. 2004 Aug 5;430(7000):694-9. doi: 10.1038/nature02794. Epub 2004 Jul 18. Nature. 2004. PMID: 15258597
-
Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation.Mol Cell Biol. 2000 Jul;20(13):4505-12. doi: 10.1128/MCB.20.13.4505-4512.2000. Mol Cell Biol. 2000. PMID: 10848577 Free PMC article.
-
Restoration of NF-kappaB activation by tumor necrosis factor alpha receptor complex-targeted MEKK3 in receptor-interacting protein-deficient cells.Mol Cell Biol. 2004 Dec;24(24):10757-65. doi: 10.1128/MCB.24.24.10757-10765.2004. Mol Cell Biol. 2004. PMID: 15572679 Free PMC article.
-
Linear ubiquitination signals in adaptive immune responses.Immunol Rev. 2015 Jul;266(1):222-36. doi: 10.1111/imr.12300. Immunol Rev. 2015. PMID: 26085218 Free PMC article. Review.
-
Positive and negative signaling components involved in TNFalpha-induced NF-kappaB activation.Cytokine. 2008 Jan;41(1):1-8. doi: 10.1016/j.cyto.2007.09.016. Cytokine. 2008. PMID: 18068998 Review.
Cited by
-
Membrane trafficking of death receptors: implications on signalling.Int J Mol Sci. 2013 Jul 11;14(7):14475-503. doi: 10.3390/ijms140714475. Int J Mol Sci. 2013. PMID: 23852022 Free PMC article. Review.
-
Classification models using circulating neutrophil transcripts can detect unruptured intracranial aneurysm.J Transl Med. 2020 Oct 15;18(1):392. doi: 10.1186/s12967-020-02550-2. J Transl Med. 2020. PMID: 33059716 Free PMC article.
-
Comprehensively surveying structure and function of RING domains from Drosophila melanogaster.PLoS One. 2011;6(9):e23863. doi: 10.1371/journal.pone.0023863. Epub 2011 Sep 2. PLoS One. 2011. PMID: 21912646 Free PMC article.
-
Cytokine Receptor Endocytosis: New Kinase Activity-Dependent and -Independent Roles of PI3K.Front Endocrinol (Lausanne). 2017 May 1;8:78. doi: 10.3389/fendo.2017.00078. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28507533 Free PMC article. Review.
-
Altering the sphingolipid acyl chain composition prevents LPS/GLN-mediated hepatic failure in mice by disrupting TNFR1 internalization.Cell Death Dis. 2013 Nov 21;4(11):e929. doi: 10.1038/cddis.2013.451. Cell Death Dis. 2013. PMID: 24263103 Free PMC article.
References
-
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. - PubMed
-
- Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. - PubMed
-
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. - PubMed
-
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. - PubMed
-
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

