Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury
- PMID: 18449604
- PMCID: PMC2504604
- DOI: 10.1007/s10162-008-0119-x
Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury
Abstract
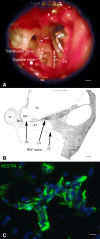
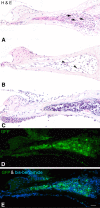
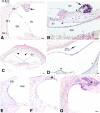
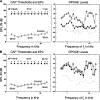
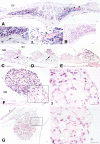
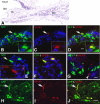
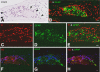
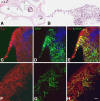
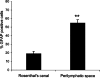
Application of ouabain to the round window membrane of the gerbil selectively induces the death of most spiral ganglion neurons and thus provides an excellent model for investigating the survival and differentiation of embryonic stem cells (ESCs) introduced into the inner ear. In this study, mouse ESCs were pretreated with a neural-induction protocol and transplanted into Rosenthal's canal (RC), perilymph, or endolymph of Mongolian gerbils either 1-3 days (early post-injury transplant group) or 7 days or longer (late post-injury transplant group) after ouabain injury. Overall, ESC survival in RC and perilymphatic spaces was significantly greater in the early post-injury microenvironment as compared to the later post-injury condition. Viable clusters of ESCs within RC and perilymphatic spaces appeared to be associated with neovascularization in the early post-injury group. A small number of ESCs transplanted within RC stained for mature neuronal or glial cell markers. ESCs introduced into perilymph survived in several locations, but most differentiated into glia-like cells. ESCs transplanted into endolymph survived poorly if at all. These experiments demonstrate that there is an optimal time window for engraftment and survival of ESCs that occurs in the early post-injury period.
Figures

Similar articles
-
Enhanced survival of bone-marrow-derived pluripotent stem cells in an animal model of auditory neuropathy.Laryngoscope. 2007 Sep;117(9):1629-35. doi: 10.1097/MLG.0b013e31806bf282. Laryngoscope. 2007. PMID: 17632425
-
Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti.J Neurobiol. 2006 Nov;66(13):1489-500. doi: 10.1002/neu.20310. J Neurobiol. 2006. PMID: 17013931 Free PMC article.
-
Transplantation of neural differentiated human mesenchymal stem cells into the cochlea of an auditory-neuropathy guinea pig model.J Korean Med Sci. 2011 Apr;26(4):492-8. doi: 10.3346/jkms.2011.26.4.492. Epub 2011 Mar 28. J Korean Med Sci. 2011. PMID: 21468255 Free PMC article.
-
Biological therapy for the inner ear.Expert Opin Biol Ther. 2004 Nov;4(11):1811-9. doi: 10.1517/14712598.4.11.1811. Expert Opin Biol Ther. 2004. PMID: 15500409 Review.
-
Cell- and gene-therapy approaches to inner ear repair.Histol Histopathol. 2011 Jul;26(7):923-40. doi: 10.14670/HH-26.923. Histol Histopathol. 2011. PMID: 21630222 Review.
Cited by
-
Challenges for stem cells to functionally repair the damaged auditory nerve.Expert Opin Biol Ther. 2013 Jan;13(1):85-101. doi: 10.1517/14712598.2013.728583. Epub 2012 Oct 25. Expert Opin Biol Ther. 2013. PMID: 23094991 Free PMC article. Review.
-
Hearing regeneration and regenerative medicine: present and future approaches.Arch Med Sci. 2019 Jul;15(4):957-967. doi: 10.5114/aoms.2019.86062. Epub 2019 Jun 20. Arch Med Sci. 2019. PMID: 31360190 Free PMC article.
-
Ouabain Does Not Induce Selective Spiral Ganglion Cell Degeneration in Guinea Pigs.Biomed Res Int. 2018 Jul 31;2018:1568414. doi: 10.1155/2018/1568414. eCollection 2018. Biomed Res Int. 2018. PMID: 30151372 Free PMC article.
-
Mechanism and Prevention of Spiral Ganglion Neuron Degeneration in the Cochlea.Front Cell Neurosci. 2022 Jan 5;15:814891. doi: 10.3389/fncel.2021.814891. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35069120 Free PMC article. Review.
-
Survival of human embryonic stem cells implanted in the guinea pig auditory epithelium.Sci Rep. 2017 Apr 7;7:46058. doi: 10.1038/srep46058. Sci Rep. 2017. PMID: 28387239 Free PMC article.
References
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0169-328X(02)00651-4', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0169-328x(02)00651-4'}, {'type': 'PubMed', 'value': '12591158', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/12591158/'}]}
- Adams LD, Choi L, Xian HQ, Yang A, Sauer B, Wei L, Gottlieb DI. Double lox targeting for neural cell transgenesis. Brain. Res. Mol. Brain Res. 110(2):220–233, 2003. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1002/cne.10244', 'is_inner': False, 'url': 'https://doi.org/10.1002/cne.10244'}, {'type': 'PubMed', 'value': '11992520', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/11992520/'}]}
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J. Comp. Neurol. 447(4):331–350, 2002. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1016/S0896-6273(04)00111-4', 'is_inner': False, 'url': 'https://doi.org/10.1016/s0896-6273(04)00111-4'}, {'type': 'PubMed', 'value': '15003168', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/15003168/'}]}
- Alvarez-Buylla A, Lim DA. For the long run: Maintaining germinal niches in the adult brain. Neuron. 41(5):683–686, 2004. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'PubMed', 'value': '9777634', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/9777634/'}]}
- Bain G, Gottlieb DI. Neural cells derived by in vitro differentiation of P19 and embryonic stem cells. Perspect. Dev. Neurobiol. 5(2–3):175–178, 1998. - PubMed
-
- {'text': '', 'ref_index': 1, 'ids': [{'type': 'DOI', 'value': '10.1006/dbio.1995.1085', 'is_inner': False, 'url': 'https://doi.org/10.1006/dbio.1995.1085'}, {'type': 'PubMed', 'value': '7729574', 'is_inner': True, 'url': 'https://pubmed.ncbi.nlm.nih.gov/7729574/'}]}
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168(2):342–357, 1995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

