Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide
- PMID: 18444645
- PMCID: PMC4163023
- DOI: 10.1021/ja710484d
Amyloid fiber formation and membrane disruption are separate processes localized in two distinct regions of IAPP, the type-2-diabetes-related peptide
Abstract
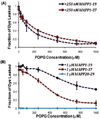
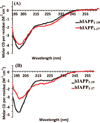
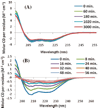
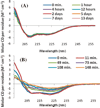
Aggregation of Islet Amyloid Polypeptide (IAPP) has been implicated in the development of type II diabetes. Because IAPP is a highly amyloidogenic peptide, it has been suggested that the formation of IAPP amyloid fibers causes disruption of the cellular membrane and is responsible for the death of beta-cells during type II diabetes. Previous studies have shown that the N-terminal 1-19 region, rather than the amyloidogenic 20-29 region, is primarily responsible for the interaction of the IAPP peptide with membranes. Liposome leakage experiments presented in this study confirm that the pathological membrane disrupting activity of the full-length hIAPP is also shared by hIAPP 1-19. The hIAPP 1-19 fragment at a low concentration of peptide induces membrane disruption to a near identical extent as the full-length peptide. At higher peptide concentrations, the hIAPP 1-19 fragment induces a greater extent of membrane disruption than the full-length peptide. Similar to the full-length peptide, hIAPP 1-19 exhibits a random coil conformation in solution and adopts an alpha-helical conformation upon binding to lipid membranes. However, unlike the full-length peptide, the hIAPP 1-19 fragment did not form amyloid fibers when incubated with POPG vesicles. These results indicate that membrane disruption can occur independently from amyloid formation in IAPP, and the sequences responsible for amyloid formation and membrane disruption are located in different regions of the peptide.
Figures

Similar articles
-
A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity.Biochemistry. 2008 Dec 2;47(48):12680-8. doi: 10.1021/bi801427c. Biochemistry. 2008. PMID: 18989933 Free PMC article.
-
Structures of rat and human islet amyloid polypeptide IAPP(1-19) in micelles by NMR spectroscopy.Biochemistry. 2008 Dec 2;47(48):12689-97. doi: 10.1021/bi8014357. Biochemistry. 2008. PMID: 18989932 Free PMC article.
-
Distinct helix propensities and membrane interactions of human and rat IAPP(1-19) monomers in anionic lipid bilayers.J Phys Chem B. 2015 Feb 26;119(8):3366-76. doi: 10.1021/jp5111357. Epub 2015 Feb 17. J Phys Chem B. 2015. PMID: 25646717
-
Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology.Biochim Biophys Acta. 2001 Nov 29;1537(3):179-203. doi: 10.1016/s0925-4439(01)00078-3. Biochim Biophys Acta. 2001. PMID: 11731221 Review.
-
The β-cell assassin: IAPP cytotoxicity.J Mol Endocrinol. 2017 Oct;59(3):R121-R140. doi: 10.1530/JME-17-0105. Epub 2017 Aug 15. J Mol Endocrinol. 2017. PMID: 28811318 Review.
Cited by
-
Melatonin Inhibits hIAPP Oligomerization by Preventing β-Sheet and Hydrogen Bond Formation of the Amyloidogenic Region Revealed by Replica-Exchange Molecular Dynamics Simulation.Int J Mol Sci. 2022 Sep 6;23(18):10264. doi: 10.3390/ijms231810264. Int J Mol Sci. 2022. PMID: 36142176 Free PMC article.
-
Chemical shift tensor - the heart of NMR: Insights into biological aspects of proteins.Prog Nucl Magn Reson Spectrosc. 2010 Aug;57(2):181-228. doi: 10.1016/j.pnmrs.2010.04.005. Epub 2010 May 7. Prog Nucl Magn Reson Spectrosc. 2010. PMID: 20633363 Free PMC article. Review. No abstract available.
-
NMR structure in a membrane environment reveals putative amyloidogenic regions of the SEVI precursor peptide PAP(248-286).J Am Chem Soc. 2009 Dec 16;131(49):17972-9. doi: 10.1021/ja908170s. J Am Chem Soc. 2009. PMID: 19995078 Free PMC article.
-
Induction of negative curvature as a mechanism of cell toxicity by amyloidogenic peptides: the case of islet amyloid polypeptide.J Am Chem Soc. 2009 Apr 1;131(12):4470-8. doi: 10.1021/ja809002a. J Am Chem Soc. 2009. PMID: 19278224 Free PMC article.
-
Nanostructural Differentiation and Toxicity of Amyloid-β25-35 Aggregates Ensue from Distinct Secondary Conformation.Sci Rep. 2018 Jan 15;8(1):765. doi: 10.1038/s41598-017-19106-y. Sci Rep. 2018. PMID: 29335442 Free PMC article.
References
-
- Hoppener JW, Nieuwenhuis MG, Vroom TM, Lips CJ. New Eng. J. Med. 2000;144:1995–2000. - PubMed
-
- Luca S, Yau W-M, Leapman R, Tycko R. Biochemistry. 2007;46:13505–13522. - PMC - PubMed
- Tycko R. Curr. Opin. Chem. Biol. 2006;4:500–506. - PubMed
- Makin OS, Serpell LC. FEBS J. 2005;272:5950–5961. - PubMed
- Mascioni A, Porcelli F, Ilangovan U, Ramamoorthy A, Venglia G. Biopolymers. 2003;69:29–41. - PubMed
-
- Konarkowska B, Aitken JF, Kistler J, Zhang SP, Cooper GJS. FEBS J. 2006;273:3614–3624. - PubMed
-
- Meier JJ, Kayed R, Lin CY, Gurlo T, Haataja L, Jayasinghe S, Langen R, Glabe CG, Butler PC. Am. J. Physiol. 2006;291:E1317–E1324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

