Modular patterning of structure and function of the striatum by retinoid receptor signaling
- PMID: 18443282
- PMCID: PMC2373312
- DOI: 10.1073/pnas.0802109105
Modular patterning of structure and function of the striatum by retinoid receptor signaling
Abstract
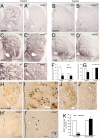
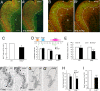
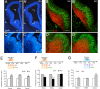
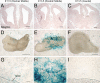
Retinoid signaling plays a crucial role in patterning rhombomeres in the hindbrain and motor neurons in the spinal cord during development. A fundamentally interesting question is whether retinoids can pattern functional organization in the forebrain that generates a high order of cognitive behavior. The striatum contains a compartmental structure of striosome (or "patch") and intervening matrix. How this highly complex mosaic design is patterned by the genetic programs during development remains elusive. We report a developmental mechanism by which retinoid receptor signaling controls compartmental formation in the striatum. We analyzed RARbeta(-/-) mutant mice and found a selective loss of striosomal compartmentalization in the rostral mutant striatum. The loss of RARbeta signaling in the mutant mice resulted in reduction of cyclin E2, a cell cycle protein regulating transition from G(1) to S phase, and also reduction of the proneural gene Mash1, which led to defective neurogenesis of late-born striosomal cells. Importantly, during striatal neurogenesis, endogenous levels of retinoic acid were spatiotemporally regulated such that transduction of high levels of retinoic acid through RARbeta selectively expanded the population of late-born striosomal progenitors, which evolved into a highly elaborate compartment in the rostral striatum. RARbeta(-/-) mutant mice, which lacked such enlarged compartment, displayed complex alternations of dopamine agonist-induced stereotypic motor behavior, including exaggeration of head bobbing movement and reduction of rearing activity. RARbeta signaling thus plays a crucial role in setting up striatal compartments that may engage in neural circuits of psychomotor control.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Genome-wide Analysis of RARβ Transcriptional Targets in Mouse Striatum Links Retinoic Acid Signaling with Huntington's Disease and Other Neurodegenerative Disorders.Mol Neurobiol. 2017 Jul;54(5):3859-3878. doi: 10.1007/s12035-016-0010-4. Epub 2016 Jul 12. Mol Neurobiol. 2017. PMID: 27405468
-
Retinoid signaling competence and RARbeta-mediated gene regulation in the developing mammalian telencephalon.Dev Dyn. 2005 Apr;232(4):887-900. doi: 10.1002/dvdy.20281. Dev Dyn. 2005. PMID: 15736225
-
Retinoic Acid Receptor β Controls Development of Striatonigral Projection Neurons through FGF-Dependent and Meis1-Dependent Mechanisms.J Neurosci. 2015 Oct 28;35(43):14467-75. doi: 10.1523/JNEUROSCI.1278-15.2015. J Neurosci. 2015. PMID: 26511239 Free PMC article.
-
Retinoic acid and hindbrain patterning.J Neurobiol. 2006 Jun;66(7):705-25. doi: 10.1002/neu.20272. J Neurobiol. 2006. PMID: 16688767 Review.
-
Diverse actions of retinoid receptors in cancer prevention and treatment.Differentiation. 2007 Nov;75(9):853-70. doi: 10.1111/j.1432-0436.2007.00206.x. Epub 2007 Jul 18. Differentiation. 2007. PMID: 17634071 Review.
Cited by
-
Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses.Nat Neurosci. 2011 Nov 27;15(2):274-83. doi: 10.1038/nn.2997. Nat Neurosci. 2011. PMID: 22119903 Free PMC article.
-
ALDH1A1 regulates postsynaptic μ-opioid receptor expression in dorsal striatal projection neurons and mitigates dyskinesia through transsynaptic retinoic acid signaling.Sci Rep. 2019 Mar 5;9(1):3602. doi: 10.1038/s41598-019-40326-x. Sci Rep. 2019. PMID: 30837649 Free PMC article.
-
Dysregulation of CalDAG-GEFI and CalDAG-GEFII predicts the severity of motor side-effects induced by anti-parkinsonian therapy.Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2892-6. doi: 10.1073/pnas.0812822106. Epub 2009 Jan 26. Proc Natl Acad Sci U S A. 2009. PMID: 19171906 Free PMC article.
-
Retinoic Acid Signaling: A New Piece in the Spoken Language Puzzle.Front Psychol. 2015 Nov 26;6:1816. doi: 10.3389/fpsyg.2015.01816. eCollection 2015. Front Psychol. 2015. PMID: 26635706 Free PMC article.
-
Developmental Characterization of Schizophrenia-Associated Gene Zswim6 in Mouse Forebrain.Front Neuroanat. 2021 May 13;15:669631. doi: 10.3389/fnana.2021.669631. eCollection 2021. Front Neuroanat. 2021. PMID: 34054439 Free PMC article.
References
-
- Graybiel AM. Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci. 1990;13:244–254. - PubMed
-
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. - PubMed
-
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: Lessons from Genetic and Pharmacological Dissections of the Retinoic Acid Signaling Pathway During Mouse Embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. - PubMed
-
- Maden M. Retinoid signalling in the development of the central nervous system. Nat Rev Neurosci. 2002;3:843–853. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

