Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation
- PMID: 18436652
- PMCID: PMC2373348
- DOI: 10.1073/pnas.0708809105
Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation
Abstract
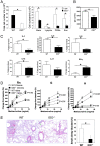
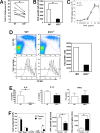
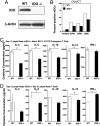
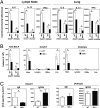
Indoleamine 2,3 dioxygenase (IDO) has emerged as an important mediator of immune tolerance via inhibition of Th1 responses. However, the role of IDO in antigen-induced tolerance or allergic inflammation in the airways that is regulated by Th2 responses has not been elucidated. By using IDO(-/-) mice, we found no impairment of airway tolerance, but, surprisingly, absence of IDO provided significant relief from establishment of allergic airways disease, as evident from attenuated Th2 cytokine production, airway inflammation, mucus secretion, airway hyperresponsiveness, and serum ovalbumin-specific IgE. Myeloid dendritic cells isolated from lung-draining lymph nodes of mice immunized for either Th1 or Th2 response revealed fewer mature dendritic cells in the lymph nodes of IDO(-/-) mice. However, the net functional impact of IDO deficiency on antigen-induced responses was more remarkable in the Th2 model than in the Th1 model. Collectively, these data suggest that IDO is not required for the induction of immune tolerance in the airways but plays a role in promoting Th2-mediated allergic airway inflammation via unique effects on lung dendritic cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Immature dendritic cells expressing indoleamine 2,3-dioxygenase suppress ovalbumin-induced allergic airway inflammation in mice.J Investig Allergol Clin Immunol. 2011;21(3):185-92. J Investig Allergol Clin Immunol. 2011. PMID: 21548446
-
Inhibition of antigen-specific immune responses by co-application of an indoleamine 2,3-dioxygenase (IDO)-encoding vector requires antigen transgene expression focused on dendritic cells.Amino Acids. 2020 Mar;52(3):411-424. doi: 10.1007/s00726-020-02817-4. Epub 2020 Feb 1. Amino Acids. 2020. PMID: 32008091
-
Intratracheal priming with ovalbumin- and ovalbumin 323-339 peptide-pulsed dendritic cells induces airway hyperresponsiveness, lung eosinophilia, goblet cell hyperplasia, and inflammation.J Immunol. 2001 Jan 15;166(2):1261-71. doi: 10.4049/jimmunol.166.2.1261. J Immunol. 2001. PMID: 11145709
-
Indoleamine-2,3-dioxygenase modulation of allergic immune responses.Curr Allergy Asthma Rep. 2006 Feb;6(1):27-31. doi: 10.1007/s11882-006-0006-7. Curr Allergy Asthma Rep. 2006. PMID: 16476191 Review.
-
IDO: a double-edged sword for T(H)1/T(H)2 regulation.Immunol Lett. 2008 Nov 16;121(1):1-6. doi: 10.1016/j.imlet.2008.08.008. Epub 2008 Sep 29. Immunol Lett. 2008. PMID: 18824197 Free PMC article. Review.
Cited by
-
Anti-regulatory T cells.Semin Immunopathol. 2017 Apr;39(3):317-326. doi: 10.1007/s00281-016-0593-x. Epub 2016 Sep 27. Semin Immunopathol. 2017. PMID: 27677755 Review.
-
Switch of NAD Salvage to de novo Biosynthesis Sustains SIRT1-RelB-Dependent Inflammatory Tolerance.Front Immunol. 2019 Oct 11;10:2358. doi: 10.3389/fimmu.2019.02358. eCollection 2019. Front Immunol. 2019. PMID: 31681271 Free PMC article.
-
Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege.Int Rev Immunol. 2010 Apr;29(2):133-55. doi: 10.3109/08830180903349669. Int Rev Immunol. 2010. PMID: 20367139 Free PMC article. Review.
-
Neurotransmitter signalling via NMDA receptors leads to decreased T helper type 1-like and enhanced T helper type 2-like immune balance in humans.Immunology. 2018 Mar;153(3):368-379. doi: 10.1111/imm.12846. Epub 2017 Nov 3. Immunology. 2018. PMID: 28940416 Free PMC article.
-
Endogenous ligands of the aryl hydrocarbon receptor regulate lung dendritic cell function.Immunology. 2016 Jan;147(1):41-54. doi: 10.1111/imm.12540. Epub 2015 Nov 10. Immunology. 2016. PMID: 26555456 Free PMC article.
References
-
- Mellor AL, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. - PubMed
-
- Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. - PubMed
-
- Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. - PubMed
-
- Beutelspacher SC, et al. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant. 2006;6:1320–1330. - PubMed
-
- Odemuyiwa SO, et al. Cutting edge: Human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

