Lipid-independent secretion of a Drosophila Wnt protein
- PMID: 18430724
- PMCID: PMC2427328
- DOI: 10.1074/jbc.M802059200
Lipid-independent secretion of a Drosophila Wnt protein
Abstract
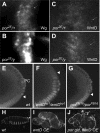
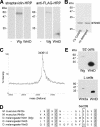
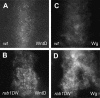
Wnt proteins comprise a large class of secreted signaling molecules with key roles during embryonic development and throughout adult life. Recently, much effort has been focused on understanding the factors that regulate Wnt signal production. For example, Porcupine and Wntless/Evi/Sprinter have been identified as being required in Wnt-producing cells for the processing and secretion of many Wnt proteins. Interestingly, in this study we find that WntD, a recently characterized Drosophila Wnt family member, does not require Porcupine or Wntless/Evi/Sprinter for its secretion or signaling activity. Because Porcupine is involved in post-translational lipid modification of Wnt proteins, we used a novel labeling method and mass spectrometry to ask whether WntD undergoes lipid modification and found that it does not. Although lipid modification is also hypothesized to be required for Wnt secretion, we find that WntD is secreted very efficiently. WntD secretion does, however, maintain a requirement for the secretory pathway component Rab1. Our results show that not all Wnt family members require lipid modification, Porcupine, or Wntless/Evi/Sprinter for secretion and suggest that different modes of secretion may exist for different Wnt proteins.
Figures

Similar articles
-
The role of Evi/Wntless in exporting Wnt proteins.Development. 2023 Feb 15;150(3):dev201352. doi: 10.1242/dev.201352. Epub 2023 Feb 10. Development. 2023. PMID: 36763105 Free PMC article. Review.
-
Porcupine-mediated lipidation is required for Wnt recognition by Wls.Dev Biol. 2012 Jan 15;361(2):392-402. doi: 10.1016/j.ydbio.2011.11.003. Epub 2011 Nov 11. Dev Biol. 2012. PMID: 22108505
-
Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells.Cell. 2006 May 5;125(3):509-22. doi: 10.1016/j.cell.2006.02.049. Cell. 2006. PMID: 16678095
-
Secretion of Wnt ligands requires Evi, a conserved transmembrane protein.Cell. 2006 May 5;125(3):523-33. doi: 10.1016/j.cell.2006.04.009. Cell. 2006. PMID: 16678096
-
A dedicated Wnt secretion factor.Cell. 2006 May 5;125(3):432-3. doi: 10.1016/j.cell.2006.04.018. Cell. 2006. PMID: 16678089 Review.
Cited by
-
The role of Evi/Wntless in exporting Wnt proteins.Development. 2023 Feb 15;150(3):dev201352. doi: 10.1242/dev.201352. Epub 2023 Feb 10. Development. 2023. PMID: 36763105 Free PMC article. Review.
-
Controlling Wnt Signaling Specificity and Implications for Targeting WNTs Pharmacologically.Handb Exp Pharmacol. 2021;269:3-28. doi: 10.1007/164_2021_529. Handb Exp Pharmacol. 2021. PMID: 34463853
-
WNT4 Balances Development vs Disease in Gynecologic Tissues and Women's Health.Endocrinology. 2021 Jul 1;162(7):bqab093. doi: 10.1210/endocr/bqab093. Endocrinology. 2021. PMID: 33963381 Free PMC article. Review.
-
A Not-So-Ancient Grease History: Click Chemistry and Protein Lipid Modifications.Chem Rev. 2021 Jun 23;121(12):7178-7248. doi: 10.1021/acs.chemrev.0c01108. Epub 2021 Apr 6. Chem Rev. 2021. PMID: 33821625 Free PMC article. Review.
-
Pluripotency state regulates cytoneme selectivity and self-organization of embryonic stem cells.J Cell Biol. 2021 Apr 5;220(4):e202005095. doi: 10.1083/jcb.202005095. J Cell Biol. 2021. PMID: 33606876 Free PMC article.
References
-
- Logan, C. Y., and Nusse, R. (2004) Annu. Rev. Cell Dev. Biol. 20 781–810 - PubMed
-
- Zecca, M., Basler, K., and Struhl, G. (1996) Cell 87 833–844 - PubMed
-
- Neumann, C. J., and Cohen, S. M. (1997) Development 124 871–880 - PubMed
-
- Kiecker, C., and Niehrs, C. (2001) Development 128 4189–4201 - PubMed
-
- Mikels, A. J., and Nusse, R. (2006) Oncogene 25 7461–7468 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

