Rab14 regulates apical targeting in polarized epithelial cells
- PMID: 18429929
- PMCID: PMC2773667
- DOI: 10.1111/j.1600-0854.2008.00752.x
Rab14 regulates apical targeting in polarized epithelial cells
Abstract
Epithelial cells display distinct apical and basolateral membrane domains, and maintenance of this asymmetry is essential to the function of epithelial tissues. Polarized delivery of apical and basolateral membrane proteins from the trans Golgi network (TGN) and/or endosomes to the correct domain requires specific cytoplasmic machinery to control the sorting, budding and fission of vesicles. However, the molecular machinery that regulates polarized delivery of apical proteins remains poorly understood. In this study, we show that the small guanosine triphosphatase Rab14 is involved in the apical targeting pathway. Using yeast two-hybrid analysis and glutathione S-transferase pull down, we show that Rab14 interacts with apical membrane proteins and localizes to the TGN and apical endosomes. Overexpression of the GDP mutant form of Rab14 (S25N) induces an enlargement of the TGN and vesicle accumulation around Golgi membranes. Moreover, expression of Rab14-S25N results in mislocalization of the apical raft-associated protein vasoactive intestinal peptide/MAL to the basolateral domain but does not disrupt basolateral targeting or recycling. These data suggest that Rab14 specifically regulates delivery of cargo from the TGN to the apical domain.
Figures


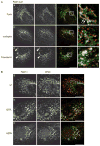
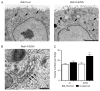
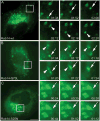
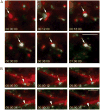

Similar articles
-
Rab13 regulates membrane trafficking between TGN and recycling endosomes in polarized epithelial cells.J Cell Biol. 2008 Sep 8;182(5):845-53. doi: 10.1083/jcb.200802176. J Cell Biol. 2008. PMID: 18779367 Free PMC article.
-
Four-dimensional live imaging of apical biosynthetic trafficking reveals a post-Golgi sorting role of apical endosomal intermediates.Proc Natl Acad Sci U S A. 2014 Mar 18;111(11):4127-32. doi: 10.1073/pnas.1304168111. Epub 2014 Mar 3. Proc Natl Acad Sci U S A. 2014. PMID: 24591614 Free PMC article.
-
Rab14 is involved in membrane trafficking between the Golgi complex and endosomes.Mol Biol Cell. 2004 May;15(5):2218-29. doi: 10.1091/mbc.e03-10-0777. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004230 Free PMC article.
-
Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane.J Cell Sci. 2004 Dec 1;117(Pt 25):5955-64. doi: 10.1242/jcs.01596. J Cell Sci. 2004. PMID: 15564373 Review.
-
Sorting of plasma membrane proteins in epithelial cells.Curr Opin Cell Biol. 1991 Aug;3(4):647-53. doi: 10.1016/0955-0674(91)90036-x. Curr Opin Cell Biol. 1991. PMID: 1772657 Review.
Cited by
-
Toxoplasma gondii salvages sphingolipids from the host Golgi through the rerouting of selected Rab vesicles to the parasitophorous vacuole.Mol Biol Cell. 2013 Jun;24(12):1974-95. doi: 10.1091/mbc.E12-11-0827. Epub 2013 Apr 24. Mol Biol Cell. 2013. PMID: 23615442 Free PMC article.
-
Rab14 from Bombyx mori (Lepidoptera: Bombycidae) shows ATPase activity.Biol Lett. 2010 Jun 23;6(3):379-81. doi: 10.1098/rsbl.2009.0878. Epub 2010 Jan 13. Biol Lett. 2010. PMID: 20071392 Free PMC article.
-
The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation.Cell Mol Gastroenterol Hepatol. 2017 Nov 15;5(2):145-156. doi: 10.1016/j.jcmgh.2017.11.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2017. PMID: 29322087 Free PMC article.
-
Rab22a regulates the establishment of epithelial polarity.Small GTPases. 2021 Jul;12(4):282-293. doi: 10.1080/21541248.2020.1754104. Epub 2020 Apr 17. Small GTPases. 2021. PMID: 32281471 Free PMC article.
-
Tribbles Pseudokinase 2 (TRIB2) Regulates Expression of Binding Partners in Bovine Granulosa Cells.Int J Mol Sci. 2021 Feb 3;22(4):1533. doi: 10.3390/ijms22041533. Int J Mol Sci. 2021. PMID: 33546420 Free PMC article.
References
-
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. - PubMed
-
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. - PubMed
-
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. - PubMed
-
- Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

