ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration
- PMID: 18426980
- PMCID: PMC2315680
- DOI: 10.1083/jcb.200709033
ErbB2 directly activates the exchange factor Dock7 to promote Schwann cell migration
Abstract
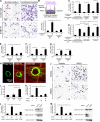
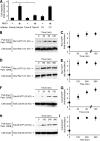
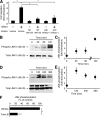
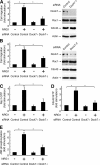
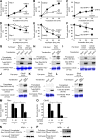
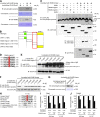
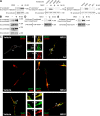
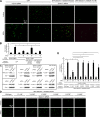
The cellular events that precede myelination in the peripheral nervous system require rapid and dynamic morphological changes in the Schwann cell. These events are thought to be mainly controlled by axonal signals. But how signals on the axons are coordinately organized and transduced to promote proliferation, migration, radial sorting, and myelination is unknown. We describe that the axonal signal neuregulin-1 (NRG1) controls Schwann cell migration via activation of the atypical Dock180-related guanine nucleotide exchange factor (GEF) Dock7 and subsequent activation of the Rho guanine triphosphatases (GTPases) Rac1 and Cdc42 and the downstream c-Jun N-terminal kinase. We show that the NRG1 receptor ErbB2 directly binds and activates Dock7 by phosphorylating Tyr-1118. Dock7 knockdown, or expression of Dock7 harboring the Tyr-1118-to-Phe mutation in Schwann cells, attenuates the effects of NRG1. Thus, Dock7 functions as an intracellular substrate for ErbB2 to promote Schwann cell migration. This provides an unanticipated mechanism through which ligand-dependent tyrosine phosphorylation can trigger the activation of Rho GTPase-GEFs of the Dock180 family.
Figures
Similar articles
-
The atypical Guanine-nucleotide exchange factor, dock7, negatively regulates schwann cell differentiation and myelination.J Neurosci. 2011 Aug 31;31(35):12579-92. doi: 10.1523/JNEUROSCI.2738-11.2011. J Neurosci. 2011. PMID: 21880919 Free PMC article.
-
Guanine nucleotide exchange factor Dock7 mediates HGF-induced glioblastoma cell invasion via Rac activation.Br J Cancer. 2014 Mar 4;110(5):1307-15. doi: 10.1038/bjc.2014.39. Epub 2014 Feb 11. Br J Cancer. 2014. PMID: 24518591 Free PMC article.
-
The neurotrophin-3 receptor TrkC directly phosphorylates and activates the nucleotide exchange factor Dbs to enhance Schwann cell migration.Proc Natl Acad Sci U S A. 2005 Apr 5;102(14):5198-203. doi: 10.1073/pnas.0501160102. Epub 2005 Mar 9. Proc Natl Acad Sci U S A. 2005. PMID: 15758069 Free PMC article.
-
Nrg1/ErbB signaling networks in Schwann cell development and myelination.Semin Cell Dev Biol. 2010 Dec;21(9):922-8. doi: 10.1016/j.semcdb.2010.08.008. Epub 2010 Sep 9. Semin Cell Dev Biol. 2010. PMID: 20832498 Free PMC article. Review.
-
Neuregulin, a factor with many functions in the life of a schwann cell.Bioessays. 2000 Nov;22(11):987-96. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. Bioessays. 2000. PMID: 11056475 Review.
Cited by
-
Manipulating oligodendrocyte intrinsic regeneration mechanism to promote remyelination.Cell Mol Life Sci. 2021 Jul;78(13):5257-5273. doi: 10.1007/s00018-021-03852-4. Epub 2021 May 21. Cell Mol Life Sci. 2021. PMID: 34019104 Free PMC article. Review.
-
EGFR ligand shifts the role of EGFR from oncogene to tumour suppressor in EGFR-amplified glioblastoma by suppressing invasion through BIN3 upregulation.Nat Cell Biol. 2022 Aug;24(8):1291-1305. doi: 10.1038/s41556-022-00962-4. Epub 2022 Aug 1. Nat Cell Biol. 2022. PMID: 35915159 Free PMC article.
-
Identification of a negative regulatory region for the exchange activity and characterization of T332I mutant of Rho guanine nucleotide exchange factor 10 (ARHGEF10).J Biol Chem. 2011 Aug 26;286(34):29511-20. doi: 10.1074/jbc.M111.236810. Epub 2011 Jun 30. J Biol Chem. 2011. PMID: 21719701 Free PMC article.
-
Postinjury Induction of Activated ErbB2 Selectively Hyperactivates Denervated Schwann Cells and Promotes Robust Dorsal Root Axon Regeneration.J Neurosci. 2017 Nov 8;37(45):10955-10970. doi: 10.1523/JNEUROSCI.0903-17.2017. Epub 2017 Oct 5. J Neurosci. 2017. PMID: 28982707 Free PMC article.
-
Optimal Objective-Based Experimental Design for Uncertain Dynamical Gene Networks with Experimental Error.IEEE/ACM Trans Comput Biol Bioinform. 2018 Jan-Feb;15(1):218-230. doi: 10.1109/TCBB.2016.2602873. Epub 2016 Aug 25. IEEE/ACM Trans Comput Biol Bioinform. 2018. PMID: 27576263 Free PMC article.
References
-
- Arthur, W.T., S.M. Ellerbroek, C.J. Der, K. Burridge, and K. Wennerberg. 2002. XPLN, a guanine nucleotide exchange factor for RhoA and RhoB, but not RhoC. J. Biol. Chem. 277:42964–42972. - PubMed
-
- Benninger, Y., T. Thurnherr, J.A. Pereira, S. Krause, X. Wu, A. Chrostek-Grashoff, D. Herzog, K.A. Nave, R.J. Franklin, D. Meijer, et al. 2007. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 177:1051–1061. - PMC - PubMed
-
- Brugnera, E., L. Haney, C. Grimsley, M. Lu, S.F. Walk, A.C. Tosello-Trampont, I.G. Macara, H. Madhani, G.R. Fink, and K.S. Ravichandran. 2002. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat. Cell Biol. 4:574–582. - PubMed
-
- Bunge, R.P. 1993. Expanding roles for the Schwann cell: ensheathment, myelination, trophism and regeneration. Curr. Opin. Neurobiol. 3:805–809. - PubMed
-
- Chan, J.R., C. Jolicoeur, J. Yamauchi, J. Elliott, J.P. Fawcett, B.K. Ng, and M. Cayouette. 2006. The polarity protein Par-3 directly interacts with the p75 neurotrophin receptor to regulate myelination. Science. 314:832–836. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

