Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor
- PMID: 18411250
- PMCID: PMC3579524
- DOI: 10.1242/jcs.020792
Dissecting the role of PtdIns(4,5)P2 in endocytosis and recycling of the transferrin receptor
Abstract
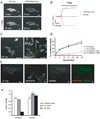
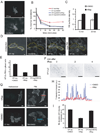
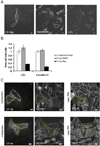
Endocytosis and recycling of membrane proteins are key processes for nutrient uptake, receptor signaling and synaptic transmission. Different steps in these fission and fusion cycles have been proposed to be regulated by physiological changes in plasma membrane (PM) phosphatidylinositol (4,5)-bisphosphate [PtdIns(4,5)P(2)] concentration. Here, we use a chemical enzyme-translocation strategy to rapidly reduce PM PtdIns(4,5)P(2) levels while monitoring clathrin-mediated endocytosis and recycling. PtdIns(4,5)P(2) hydrolysis blocked transferrin receptor endocytosis and led to a marked increase in the concentration of transferrin receptors in the PM, suggesting that endocytosis is more sensitive to changes in PtdIns(4,5)P(2) than recycling. Reduction of PM PtdIns(4,5)P(2) levels led to a near complete dissociation of Adaptor protein 2 (AP-2) from the PM but had only a small effect on clathrin assembly. This argues that receptor-mediated PtdIns(4,5)P(2) reduction preferentially suppresses AP-2-mediated targeting of cargo to endocytic sites rather than the assembly of clathrin coats or recycling of endocytic vesicles.
Figures

Similar articles
-
Clathrin-mediated endocytosis in AP-2-depleted cells.J Cell Biol. 2003 Sep 1;162(5):909-18. doi: 10.1083/jcb.200305145. J Cell Biol. 2003. PMID: 12952941 Free PMC article.
-
Phosphatidylinositol-4,5-bisphosphate-rich plasma membrane patches organize active zones of endocytosis and ruffling in cultured adipocytes.Mol Cell Biol. 2004 Oct;24(20):9102-23. doi: 10.1128/MCB.24.20.9102-9123.2004. Mol Cell Biol. 2004. PMID: 15456883 Free PMC article.
-
Distinct roles for plasma membrane PtdIns(4)P and PtdIns(4,5)P2 during receptor-mediated endocytosis in yeast.J Cell Sci. 2018 Jan 4;131(1):jcs207696. doi: 10.1242/jcs.207696. J Cell Sci. 2018. PMID: 29192062
-
Phosphoinositide regulation of clathrin-mediated endocytosis.Biochem Soc Trans. 2005 Dec;33(Pt 6):1285-9. doi: 10.1042/BST0331285. Biochem Soc Trans. 2005. PMID: 16246100 Review.
-
Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review.
Cited by
-
Astroglial IFITM3 mediates neuronal impairments following neonatal immune challenge in mice.Glia. 2013 May;61(5):679-93. doi: 10.1002/glia.22461. Epub 2013 Feb 4. Glia. 2013. PMID: 23382131 Free PMC article.
-
Reorganization of Ternary Lipid Mixtures of Nonphosphorylated Phosphatidylinositol Interacting with Angiomotin.J Phys Chem B. 2018 Sep 6;122(35):8404-8415. doi: 10.1021/acs.jpcb.7b12641. Epub 2018 Aug 27. J Phys Chem B. 2018. PMID: 29877706 Free PMC article.
-
Transmembrane protein 55B is a novel regulator of cellular cholesterol metabolism.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):1917-23. doi: 10.1161/ATVBAHA.113.302806. Epub 2014 Jul 17. Arterioscler Thromb Vasc Biol. 2014. PMID: 25035345 Free PMC article.
-
Annotating MYC status with 89Zr-transferrin imaging.Nat Med. 2012 Oct;18(10):1586-91. doi: 10.1038/nm.2935. Epub 2012 Sep 23. Nat Med. 2012. PMID: 23001181 Free PMC article.
-
Identification of postsynaptic phosphatidylinositol-4,5-bisphosphate (PIP2) roles for synaptic plasticity using chemically induced dimerization.Sci Rep. 2017 Jun 13;7(1):3351. doi: 10.1038/s41598-017-03520-3. Sci Rep. 2017. PMID: 28611378 Free PMC article.
References
-
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 2001;17:517–568. - PubMed
-
- Cremona O, De Camilli P. Phosphoinositides in membrane traffic at the synapse. J. Cell Sci. 2001;114:1041–1451. - PubMed
-
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. - PubMed
-
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

