Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism
- PMID: 18391946
- PMCID: PMC2532986
- DOI: 10.1038/nn.2100
Oligomeric amyloid-beta peptide disrupts phosphatidylinositol-4,5-bisphosphate metabolism
Abstract
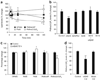
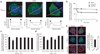
Synaptic dysfunction caused by oligomeric assemblies of amyloid-beta peptide (Abeta) has been linked to cognitive deficits in Alzheimer's disease. Here we found that incubation of primary cortical neurons with oligomeric Abeta decreases the level of phosphatidylinositol-4,5-bisphosphate (PtdIns(4,5)P2), a phospholipid that regulates key aspects of neuronal function. The destabilizing effect of Abeta on PtdIns(4,5)P2 metabolism was Ca2+-dependent and was not observed in neurons that were derived from mice that are haploinsufficient for Synj1. This gene encodes synaptojanin 1, the main PtdIns(4,5)P2 phosphatase in the brain and at the synapses. We also found that the inhibitory effect of Abeta on hippocampal long-term potentiation was strongly suppressed in slices from Synj1+/- mice, suggesting that Abeta-induced synaptic dysfunction can be ameliorated by treatments that maintain the normal PtdIns(4,5)P2 balance in the brain.
Figures
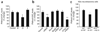
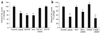

Similar articles
-
Reduction of synaptojanin 1 accelerates Aβ clearance and attenuates cognitive deterioration in an Alzheimer mouse model.J Biol Chem. 2013 Nov 1;288(44):32050-63. doi: 10.1074/jbc.M113.504365. Epub 2013 Sep 19. J Biol Chem. 2013. PMID: 24052255 Free PMC article.
-
Reduction of synaptojanin 1 ameliorates synaptic and behavioral impairments in a mouse model of Alzheimer's disease.J Neurosci. 2012 Oct 31;32(44):15271-6. doi: 10.1523/JNEUROSCI.2034-12.2012. J Neurosci. 2012. PMID: 23115165 Free PMC article.
-
Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down's syndrome.Proc Natl Acad Sci U S A. 2008 Jul 8;105(27):9415-20. doi: 10.1073/pnas.0803756105. Epub 2008 Jun 30. Proc Natl Acad Sci U S A. 2008. PMID: 18591654 Free PMC article.
-
Regulation of phosphatidylinositol 4,5-bisphosphate levels and its roles in cytoskeletal re-organization and malignant transformation.Chem Phys Lipids. 1999 Apr;98(1-2):13-22. doi: 10.1016/s0009-3084(99)00014-6. Chem Phys Lipids. 1999. PMID: 10358924 Review.
-
Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms.Biomed Res Int. 2014;2014:289728. doi: 10.1155/2014/289728. Epub 2014 Sep 16. Biomed Res Int. 2014. PMID: 25302295 Free PMC article. Review.
Cited by
-
Molecular and cellular alterations in Down syndrome: toward the identification of targets for therapeutics.Neural Plast. 2012;2012:171639. doi: 10.1155/2012/171639. Epub 2012 Jul 12. Neural Plast. 2012. PMID: 22848846 Free PMC article. Review.
-
Reversal of cerebral hypoperfusion: a novel therapeutic target for the treatment of AD/ADRD?Geroscience. 2021 Apr;43(2):1065-1067. doi: 10.1007/s11357-021-00357-7. Epub 2021 Mar 27. Geroscience. 2021. PMID: 33772733 Free PMC article.
-
Cholesterol modulates ion channels via down-regulation of phosphatidylinositol 4,5-bisphosphate.J Neurochem. 2010 Mar;112(5):1286-94. doi: 10.1111/j.1471-4159.2009.06545.x. Epub 2009 Dec 14. J Neurochem. 2010. PMID: 20015154 Free PMC article.
-
Phospholipase D in brain function and Alzheimer's disease.Biochim Biophys Acta. 2010 Aug;1801(8):799-805. doi: 10.1016/j.bbalip.2010.04.004. Epub 2010 Apr 23. Biochim Biophys Acta. 2010. PMID: 20399893 Free PMC article. Review.
-
Amyloid β oligomers suppress excitatory transmitter release via presynaptic depletion of phosphatidylinositol-4,5-bisphosphate.Nat Commun. 2019 Mar 13;10(1):1193. doi: 10.1038/s41467-019-09114-z. Nat Commun. 2019. PMID: 30867420 Free PMC article.
References
-
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. - PubMed
-
- Haass C, Selkoe DJ. D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid-β peptide. Nat. Rev. Mol. Cell Biol. 2007;8:101–112. - PubMed
-
- Marjaux E, Hartmann D, De Strooper B. Presenilins inmemory, Alzheimer's disease and therapy. Neuron. 2004;42:189–192. - PubMed
-
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
-
- Snyder EM, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 2005;8:1051–1058. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R21 HD047733-01A1/HD/NICHD NIH HHS/United States
- R01 NS056049-01/NS/NINDS NIH HHS/United States
- NS056049/NS/NINDS NIH HHS/United States
- R01 AT002643/AT/NCCIH NIH HHS/United States
- R01 NS049442/NS/NINDS NIH HHS/United States
- R01 NS043467/NS/NINDS NIH HHS/United States
- AT002643/AT/NCCIH NIH HHS/United States
- NS049442/NS/NINDS NIH HHS/United States
- R21 HD047733-02/HD/NICHD NIH HHS/United States
- HD047733/HD/NICHD NIH HHS/United States
- R01 NS056049/NS/NINDS NIH HHS/United States
- R21 HD047733/HD/NICHD NIH HHS/United States
- R01 NS056049-02/NS/NINDS NIH HHS/United States
- NS043467/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

