Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C)
- PMID: 18382652
- PMCID: PMC2268738
- DOI: 10.1371/journal.pone.0001847
Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C)
Abstract
Background: Human rhinoviruses (HRVs) are the most frequently detected pathogens in acute respiratory tract infections (ARTIs) and yet little is known about the prevalence, recurrence, structure and clinical impact of individual members. During 2007, the complete coding sequences of six previously unknown and highly divergent HRV strains were reported. To catalogue the molecular and clinical features distinguishing the divergent HRV strains, we undertook, for the first time, in silico analyses of all available polyprotein sequences and performed retrospective reviews of the medical records of cases in which variants of the prototype strain, HRV-QPM, had been detected.
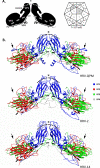
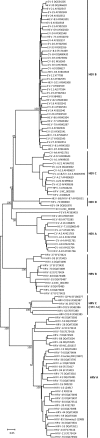
Methodology/principle findings: Genomic analyses revealed that the six divergent strains, residing within a clade we previously called HRV A2, had the shortest polyprotein of all picornaviruses investigated. Structure-based amino acid alignments identified conserved motifs shared among members of the genus Rhinovirus as well as substantive deletions and insertions unique to the divergent strains. Deletions mostly affected regions encoding proteins traditionally involved in antigenicity and serving as HRV and HEV receptor footprints. Because the HRV A2 strains cannot yet be cultured, we created homology models of predicted HRV-QPM structural proteins. In silico comparisons confirmed that HRV-QPM was most closely related to the major group HRVs. HRV-QPM was most frequently detected in infants with expiratory wheezing or persistent cough who had been admitted to hospital and required supplemental oxygen. It was the only virus detected in 65% of positive individuals. These observations contributed to an objective clinical impact ranging from mild to severe.
Conclusions: The divergent strains did not meet classification requirements for any existing species of the genus Rhinovirus or Enterovirus. HRV A2 strains should be partitioned into at least one new species, putatively called Human rhinovirus C, populated by members detected with high frequency, from individuals with respiratory symptoms requiring hospital admission.
Conflict of interest statement
Figures




Similar articles
-
Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis.J Clin Virol. 2007 Jun;39(2):67-75. doi: 10.1016/j.jcv.2007.03.012. Epub 2007 May 7. J Clin Virol. 2007. PMID: 17482871 Free PMC article.
-
Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses.J Gen Virol. 2005 Mar;86(Pt 3):697-706. doi: 10.1099/vir.0.80445-0. J Gen Virol. 2005. PMID: 15722530
-
Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children.J Clin Microbiol. 2007 Nov;45(11):3655-64. doi: 10.1128/JCM.01254-07. Epub 2007 Sep 5. J Clin Microbiol. 2007. PMID: 17804649 Free PMC article.
-
Newly identified human rhinoviruses: molecular methods heat up the cold viruses.Rev Med Virol. 2010 May;20(3):156-76. doi: 10.1002/rmv.644. Rev Med Virol. 2010. PMID: 20127751 Free PMC article. Review.
-
Proposals for the classification of human rhinovirus species C into genotypically assigned types.J Gen Virol. 2010 Oct;91(Pt 10):2409-19. doi: 10.1099/vir.0.023994-0. Epub 2010 Jul 7. J Gen Virol. 2010. PMID: 20610666 Review.
Cited by
-
Update on Human Rhinovirus and Coronavirus Infections.Semin Respir Crit Care Med. 2016 Aug;37(4):555-71. doi: 10.1055/s-0036-1584797. Epub 2016 Aug 3. Semin Respir Crit Care Med. 2016. PMID: 27486736 Free PMC article. Review.
-
Personal Network Inference Unveils Heterogeneous Immune Response Patterns to Viral Infection in Children with Acute Wheezing.J Pers Med. 2021 Dec 3;11(12):1293. doi: 10.3390/jpm11121293. J Pers Med. 2021. PMID: 34945765 Free PMC article.
-
Prevalence and clinical characterization of a newly identified human rhinovirus C species in children with acute respiratory tract infections.J Clin Microbiol. 2009 Sep;47(9):2895-900. doi: 10.1128/JCM.00745-09. Epub 2009 Jul 22. J Clin Microbiol. 2009. PMID: 19625482 Free PMC article.
-
ICAM-1 Binding Rhinoviruses Enter HeLa Cells via Multiple Pathways and Travel to Distinct Intracellular Compartments for Uncoating.Viruses. 2017 Apr 1;9(4):68. doi: 10.3390/v9040068. Viruses. 2017. PMID: 28368306 Free PMC article.
-
Clinical and molecular features of human rhinovirus C.Microbes Infect. 2012 Jun;14(6):485-94. doi: 10.1016/j.micinf.2011.12.011. Epub 2012 Jan 12. Microbes Infect. 2012. PMID: 22285901 Free PMC article. Review.
References
-
- Kiang D, Yagi S, Kantardjieff KA, Kim EJ, Louie JK, et al. Molecular characterization of a variant rhinovirus from an outbreak associated with uncommonly high mortality. J Clin Virol. 2007;38:227–237. - PubMed
-
- Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, et al. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

