DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory
- PMID: 18373436
- PMCID: PMC2677999
- DOI: 10.1089/aid.2007.0191
DNA-MVA vaccine protection after X4 SHIV challenge in macaques correlates with day-of-challenge antiviral CD4+ cell-mediated immunity levels and postchallenge preservation of CD4+ T cell memory
Abstract
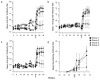
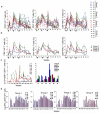
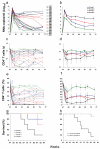
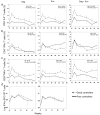
The ability of vaccines to induce immunity both in mucosal and systemic compartments may be required for prevention of HIV infection and AIDS. We compared DNA-MVA vaccination regimens adjuvanted by IL-12 DNA, administered intramuscularly and nasally or only nasally. Most of the vaccinated Rhesus macaques developed mucosal and systemic humoral and cell-mediated SHIV-specific immune responses. Stimulation of mucosal anti-Env IgA responses was limited. After rectal challenge with SHIV 89.6P, all vaccinated and naive animals became infected. However, most of the vaccinated animals showed significant control of viremia and protection from CD4(+) T cell loss and AIDS progression compared to the control animals. The levels of CD4(+) and CD8(+) T cell virus-specific responses measured on the day of challenge correlated with the level of viremia control observed later during the chronic infection. Postchallenge viremia levels inversely correlated with the preservation of SHIV-specific CD4(+)/IL-2(+) and CD8(+)/TNF-alpha(+) T cells but not with CD4(+)/IFN-gamma(+) T cells measured over time after challenge. We also found that during the early chronic infection SHIV vaccination permitted a more significant preservation of both naive and memory CD4(+) T cells compared to controls. In addition, we observed a more significant and prolonged preservation of memory CD4(+) T cells after SHIV vaccination and challenge than that observed after SIV vaccination and challenge. As the antiviral immunity stimulated by vaccination is present in the memory CD4(+) T cell subpopulations, its more limited targeting by SHIV compared to SIV may explain the better control of X4 tropic SHIV than R5 tropic SIVs by vaccination.
Figures

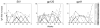
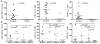
Similar articles
-
Control of simian/human immunodeficiency virus viremia and disease progression after IL-2-augmented DNA-modified vaccinia virus Ankara nasal vaccination in nonhuman primates.J Immunol. 2004 Mar 15;172(6):3745-57. doi: 10.4049/jimmunol.172.6.3745. J Immunol. 2004. PMID: 15004179
-
Long-term control of simian immunodeficiency virus mac251 viremia to undetectable levels in half of infected female rhesus macaques nasally vaccinated with simian immunodeficiency virus DNA/recombinant modified vaccinia virus Ankara.J Immunol. 2011 Mar 15;186(6):3581-93. doi: 10.4049/jimmunol.1002594. Epub 2011 Feb 11. J Immunol. 2011. PMID: 21317390
-
CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge.J Virol. 2014 Sep 1;88(17):9579-89. doi: 10.1128/JVI.00975-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920805 Free PMC article.
-
Antiviral CD8+ T cells in the genital tract control viral replication and delay progression to AIDS after vaginal SIV challenge in rhesus macaques immunized with virulence attenuated SHIV 89.6.J Intern Med. 2009 Jan;265(1):67-77. doi: 10.1111/j.1365-2796.2008.02051.x. J Intern Med. 2009. PMID: 19093961 Free PMC article. Review.
-
Mucosal immunity and protection against HIV/SIV infection: strategies and challenges for vaccine design.Int Rev Immunol. 2009;28(1):20-48. doi: 10.1080/08830180802684331. Int Rev Immunol. 2009. PMID: 19241252 Free PMC article. Review.
Cited by
-
Antibodies to gp120 and PD-1 expression on virus-specific CD8+ T cells in protection from simian AIDS.J Virol. 2013 Mar;87(6):3526-37. doi: 10.1128/JVI.02686-12. Epub 2013 Jan 16. J Virol. 2013. PMID: 23325679 Free PMC article.
-
Use of nonhuman primate models to develop mucosal AIDS vaccines.Curr HIV/AIDS Rep. 2010 Feb;7(1):19-27. doi: 10.1007/s11904-009-0035-7. Curr HIV/AIDS Rep. 2010. PMID: 20425054 Free PMC article. Review.
-
Inclusion of a CRF01_AE HIV envelope protein boost with a DNA/MVA prime-boost vaccine: Impact on humoral and cellular immunogenicity and viral load reduction after SHIV-E challenge.Vaccine. 2012 Feb 27;30(10):1830-40. doi: 10.1016/j.vaccine.2011.12.131. Epub 2012 Jan 9. Vaccine. 2012. PMID: 22234262 Free PMC article.
-
Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations.J Gen Virol. 2011 Jan;92(Pt 1):128-40. doi: 10.1099/vir.0.023242-0. J Gen Virol. 2011. PMID: 21169215 Free PMC article.
-
Challenges in mucosal HIV vaccine development: lessons from non-human primate models.Viruses. 2014 Aug 15;6(8):3129-58. doi: 10.3390/v6083129. Viruses. 2014. PMID: 25196380 Free PMC article. Review.
References
-
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. - PubMed
-
- Robinson HL. New hope for an AIDS vaccine. Nat Rev Immunol. 2002;2:239–250. - PubMed
-
- Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, Zhang ZQ, Simon AJ, Trigona WL, Dubey SA, Huang L, Harris VA, Long RS, Liang X, Handt L, Schleif WA, Zhu L, Freed DC, Persaud NV, Guan L, Punt KS, Tang A, Chen M, Wilson KA, Collins KB, Heidecker GJ, Fernandez VR, Perry HC, Joyce JG, Grimm KM, Cook JC, Keller PM, Kresock DS, Mach H, Troutman RD, Isopi LA, Williams DM, Xu Z, Bohannon KE, Volkin DB, Montefiori DC, Miura A, Krivulka GR, Lifton MA, Kuroda MJ, Schmitz JE, Letvin NL, Caulfield MJ, Bett AJ, Youil R, Kaslow DC, Emini EA. Replication-incompetent adenoviral vaccine vector elicits effective antiimmunodeficiency-virus immunity. Nature. 2002;415:331–335. - PubMed
-
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O'Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79:15547–15555. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

