Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from fas-mediated apoptosis
- PMID: 18356280
- PMCID: PMC2453085
- DOI: 10.1210/en.2008-0080
Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from fas-mediated apoptosis
Abstract
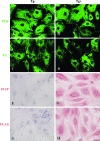
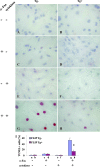
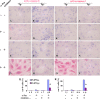
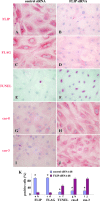
The antiapoptotic molecule Fas-associated death domain-like IL-1beta-converting enzyme inhibitory protein (FLIP) inhibits Fas-mediated apoptosis by blocking activation of caspase-8. We previously showed that expression of transgenic FLIP on thyroid epithelial cells (TECs) of DBA/1 and CBA/J mice promoted earlier resolution of granulomatous experimental autoimmune thyroiditis in vivo. This study was undertaken to directly determine whether transgenic FLIP expressed on cultured TECs can protect TECs from Fas-mediated apoptosis in vitro. The results indicate that cultured TECs from DBA/1 and CBA/J mice can be sensitized in vitro by interferon-gamma and TNF-alpha to undergo Fas-mediated apoptosis. Transgenic overexpression of FLIP protected cultured TECs of FLIP transgene (Tg)+ DBA/1 and CBA/J mice from Fas-mediated apoptosis, and FLIP small interfering RNA transfection of cultured TECs of FLIP Tg+ DBA/1 and CBA/J mice abolished the protective effect. These in vitro results are consistent with our previous in vivo studies using DBA/1 and CBA/J FLIP Tg+ mice and provide direct support for the hypothesis that transgenic expression of FLIP promotes resolution of granulomatous experimental autoimmune thyroiditis by protecting TECs from apoptosis.
Figures
Similar articles
-
Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice.Endocrinology. 2007 Dec;148(12):5734-45. doi: 10.1210/en.2007-0939. Epub 2007 Sep 6. Endocrinology. 2007. PMID: 17823262
-
Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice.Am J Pathol. 2007 Mar;170(3):875-87. doi: 10.2353/ajpath.2007.060816. Am J Pathol. 2007. PMID: 17322373 Free PMC article.
-
Effect of transgenic overexpression of FLIP on lymphocytes on development and resolution of experimental autoimmune thyroiditis.Am J Pathol. 2011 Sep;179(3):1211-20. doi: 10.1016/j.ajpath.2011.05.054. Epub 2011 Jul 16. Am J Pathol. 2011. PMID: 21763264 Free PMC article.
-
Regulation of extrinsic apoptotic signaling by c-FLIP: towards targeting cancer networks.Trends Cancer. 2022 Mar;8(3):190-209. doi: 10.1016/j.trecan.2021.12.002. Epub 2021 Dec 29. Trends Cancer. 2022. PMID: 34973957 Review.
-
Proliferative versus apoptotic functions of caspase-8 Hetero or homo: the caspase-8 dimer controls cell fate.Biochim Biophys Acta. 2012 Jan;1824(1):113-22. doi: 10.1016/j.bbapap.2011.06.005. Epub 2011 Jun 16. Biochim Biophys Acta. 2012. PMID: 21704196 Free PMC article. Review.
Cited by
-
IL-33 acts as a foe to MIA PaCa-2 pancreatic cancer.Med Oncol. 2017 Feb;34(2):23. doi: 10.1007/s12032-016-0880-3. Epub 2017 Jan 5. Med Oncol. 2017. PMID: 28058630
-
Comparison of sensitivity of Th1, Th2, and Th17 cells to Fas-mediated apoptosis.J Leukoc Biol. 2010 Jun;87(6):1019-28. doi: 10.1189/jlb.0509352. J Leukoc Biol. 2010. PMID: 20179154 Free PMC article.
-
TGF-β promotes proliferation of thyroid epithelial cells in IFN-γ(-/-) mice by down-regulation of p21 and p27 via AKT pathway.Am J Pathol. 2012 Feb;180(2):650-60. doi: 10.1016/j.ajpath.2011.10.009. Epub 2011 Nov 24. Am J Pathol. 2012. PMID: 22119715 Free PMC article.
-
CD8+ T cells induce thyroid epithelial cell hyperplasia and fibrosis.J Immunol. 2011 Feb 15;186(4):2655-62. doi: 10.4049/jimmunol.1002884. Epub 2011 Jan 10. J Immunol. 2011. PMID: 21220693 Free PMC article.
-
Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis.Cancer Sci. 2012 Jun;103(6):1090-8. doi: 10.1111/j.1349-7006.2012.02272.x. Epub 2012 Apr 15. Cancer Sci. 2012. PMID: 22417066 Free PMC article.
References
-
- Wajant H 2002 The Fas signaling pathway: more than a paradigm. Science 296:1635–1636 - PubMed
-
- Thompson CB 1995 Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462 - PubMed
-
- Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A 1995 Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373:444–448 - PubMed
-
- Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA 1995 Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 270:1189–1192 - PubMed
-
- Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima SI, Sameshima M, Hase A, Seto Y, Nagata S 1991 The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 66:233–243 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

