Reprogramming mRNA translation during stress
- PMID: 18356035
- PMCID: PMC2841789
- DOI: 10.1016/j.ceb.2008.01.013
Reprogramming mRNA translation during stress
Abstract
The survival of mammalian cells exposed to adverse environmental conditions requires a radical reprogramming of protein translation. Stress-activated kinases target components of the initiation machinery (e.g. eIF2alpha, eIF4E-BP, eIF4B, and ribosomal protein S6) to inhibit the translation of 'housekeeping' proteins and promote the translation of repair enzymes. Accumulating untranslated mRNA is concentrated at stress granules where it is sorted and triaged to sites of storage, reinitiation, or decay. At the same time, the translation of mRNAs encoding repair enzymes is selectively preserved by both internal ribosome entry site-dependent and -independent mechanisms. In combination, these stress-activated processes coordinately reprogram mRNA translation and decay in a way that conserves anabolic energy, preserves essential mRNAs, and promotes the repair of stress-induced molecular damage.
Figures

 indicates the possible SG-related mechanism.
indicates the possible SG-related mechanism.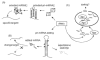
Similar articles
-
Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6.J Biol Chem. 1999 Apr 23;274(17):11647-52. doi: 10.1074/jbc.274.17.11647. J Biol Chem. 1999. PMID: 10206976
-
Interaction of translation initiation factor eIF4B with the poliovirus internal ribosome entry site.J Virol. 2002 Mar;76(5):2113-22. doi: 10.1128/jvi.76.5.2113-2122.2002. J Virol. 2002. PMID: 11836388 Free PMC article.
-
Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha.J Biol Chem. 2002 May 24;277(21):19198-205. doi: 10.1074/jbc.M201052200. Epub 2002 Mar 4. J Biol Chem. 2002. PMID: 11877448
-
Untranslated regions (UTRs) orchestrate translation reprogramming in cellular stress responses.J Therm Biol. 2017 Apr;65:69-75. doi: 10.1016/j.jtherbio.2017.02.006. Epub 2017 Feb 16. J Therm Biol. 2017. PMID: 28343578 Review.
-
Regulation of human immunodeficiency virus type 1 (HIV-1) mRNA translation.Biochem Soc Trans. 2017 Apr 15;45(2):353-364. doi: 10.1042/BST20160357. Biochem Soc Trans. 2017. PMID: 28408475 Review.
Cited by
-
Extracellular vesicle-depleted fetal bovine and human sera have reduced capacity to support cell growth.J Extracell Vesicles. 2015 Mar 26;4:26373. doi: 10.3402/jev.v4.26373. eCollection 2015. J Extracell Vesicles. 2015. PMID: 25819213 Free PMC article.
-
RNA Granules and Cataract.Expert Rev Ophthalmol. 2011 Oct 1;6(5):497-500. doi: 10.1586/eop.11.53. Expert Rev Ophthalmol. 2011. PMID: 23847690 Free PMC article. No abstract available.
-
YB-1 regulates tiRNA-induced Stress Granule formation but not translational repression.Nucleic Acids Res. 2016 Aug 19;44(14):6949-60. doi: 10.1093/nar/gkw418. Epub 2016 May 12. Nucleic Acids Res. 2016. PMID: 27174937 Free PMC article.
-
The Role of Stress Granules in the Neuronal Differentiation of Stem Cells.Mol Cells. 2020 Oct 31;43(10):848-855. doi: 10.14348/molcells.2020.0135. Mol Cells. 2020. PMID: 33028745 Free PMC article.
-
Molecular mechanisms of mTOR regulation by stress.Mol Cell Oncol. 2014 Dec 3;2(2):e970489. doi: 10.4161/23723548.2014.970489. eCollection 2015 Apr-Jun. Mol Cell Oncol. 2014. PMID: 27308421 Free PMC article. Review.
References
-
- Sorensen JG, Loeschcke V. Studying stress responses in the post-genomic era: its ecological and evolutionary role. J Biosci. 2007;32:447–456. - PubMed
-
- Brostrom CO, Brostrom MA. Regulation of translational initiation during cellular responses to stress. Prog Nucleic Acid Res Mol Biol. 1998;58:79–125. - PubMed
-
- Keene JD. RNA regulons: coordination of post-transcriptional events. Nat Rev Genet. 2007;8:533–543. A comprehensive review about mRNA regulation by RNA binding proteins. The author describes their roles in splicing, nuclear export, stability, localization and translation of mRNA. - PubMed
-
- Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–1518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

