The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS
- PMID: 18354494
- PMCID: PMC2323256
- DOI: 10.1038/emboj.2008.59
The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS
Abstract
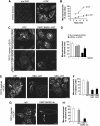
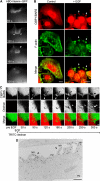
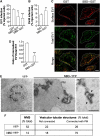
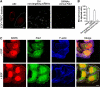
Membrane fission is an essential process in membrane trafficking and other cellular functions. While many fissioning and trafficking steps are mediated by the large GTPase dynamin, some fission events are dynamin independent and involve C-terminal-binding protein-1/brefeldinA-ADP ribosylated substrate (CtBP1/BARS). To gain an insight into the molecular mechanisms of CtBP1/BARS in fission, we have studied the role of this protein in macropinocytosis, a dynamin-independent endocytic pathway that can be synchronously activated by growth factors. Here, we show that upon activation of the epidermal growth factor receptor, CtBP1/BARS is (a) translocated to the macropinocytic cup and its surrounding membrane, (b) required for the fission of the macropinocytic cup and (c) phosphorylated on a specific serine that is a substrate for p21-activated kinase, with this phosphorylation being essential for the fission of the macropinocytic cup. Importantly, we also show that CtBP1/BARS is required for macropinocytic internalization and infection of echovirus 1. These results provide an insight into the molecular mechanisms of CtBP1/BARS activation in membrane fissioning, and extend the relevance of CtBP1/BARS-induced fission to human viral infection.
Figures

Similar articles
-
Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3.EMBO J. 2008 Apr 9;27(7):956-69. doi: 10.1038/emboj.2008.38. Epub 2008 Mar 6. EMBO J. 2008. PMID: 18323776 Free PMC article.
-
CtBP1/BARS is an activator of phospholipase D1 necessary for agonist-induced macropinocytosis.EMBO J. 2009 May 6;28(9):1197-207. doi: 10.1038/emboj.2009.78. Epub 2009 Mar 26. EMBO J. 2009. PMID: 19322195 Free PMC article.
-
Identification and characterization of a new potent inhibitor targeting CtBP1/BARS in melanoma cells.J Exp Clin Cancer Res. 2024 May 6;43(1):137. doi: 10.1186/s13046-024-03044-5. J Exp Clin Cancer Res. 2024. PMID: 38711119 Free PMC article.
-
Components of the CtBP1/BARS-dependent fission machinery.Histochem Cell Biol. 2013 Oct;140(4):407-21. doi: 10.1007/s00418-013-1138-1. Epub 2013 Sep 1. Histochem Cell Biol. 2013. PMID: 23996193 Review.
-
Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides.J Cell Mol Med. 2007 Jul-Aug;11(4):670-84. doi: 10.1111/j.1582-4934.2007.00062.x. J Cell Mol Med. 2007. PMID: 17760832 Free PMC article. Review.
Cited by
-
SP-R210 isoforms of Myosin18A modulate endosomal sorting and recognition of influenza A virus infection in macrophages.Microbes Infect. 2024 Mar-Apr;26(3):105280. doi: 10.1016/j.micinf.2023.105280. Epub 2023 Dec 21. Microbes Infect. 2024. PMID: 38135024
-
Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling.J Cell Biol. 2010 Feb 22;188(4):547-63. doi: 10.1083/jcb.200908086. Epub 2010 Feb 15. J Cell Biol. 2010. PMID: 20156964 Free PMC article.
-
Exosomal transmission of viruses, a two-edged biological sword.Cell Commun Signal. 2023 Jan 23;21(1):19. doi: 10.1186/s12964-022-01037-5. Cell Commun Signal. 2023. PMID: 36691072 Free PMC article. Review.
-
RNAi-Based Approaches for Pancreatic Cancer Therapy.Pharmaceutics. 2021 Oct 8;13(10):1638. doi: 10.3390/pharmaceutics13101638. Pharmaceutics. 2021. PMID: 34683931 Free PMC article. Review.
-
Secreted protein acidic and rich in cysteine internalization and its age-related alterations in skeletal muscle progenitor cells.Aging Cell. 2014 Feb;13(1):175-84. doi: 10.1111/acel.12168. Epub 2013 Nov 19. Aging Cell. 2014. PMID: 24245505 Free PMC article.
References
-
- Ahram M, Sameni M, Qiu RG, Linebaugh B, Kirn D, Sloane BF (2000) Rac1-induced endocytosis is associated with intracellular proteolysis during migration through a three-dimensional matrix. Exp Cell Res 260: 292–303 - PubMed
-
- Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ (2002) Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol 291: 487–494 - PubMed
-
- Barnes CJ, Vadlamudi RK, Mishra SK, Jacobson RH, Li F, Kumar R (2003) Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat Struct Biol 10: 622–628 - PubMed
-
- Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A (2005) CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol 7: 570–580 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

