Nitric oxide antagonizes the acid tolerance response that protects Salmonella against innate gastric defenses
- PMID: 18350168
- PMCID: PMC2266805
- DOI: 10.1371/journal.pone.0001833
Nitric oxide antagonizes the acid tolerance response that protects Salmonella against innate gastric defenses
Abstract
Background: Reactive nitrogen species (RNS) derived from dietary and salivary inorganic nitrogen oxides foment innate host defenses associated with the acidity of the stomach. The mechanisms by which these reactive species exert antimicrobial activity in the gastric lumen are, however, poorly understood.
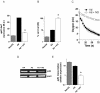
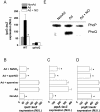
Methodology/principal findings: The genetically tractable acid tolerance response (ATR) that enables enteropathogens to survive harsh acidity was screened for signaling pathways responsive to RNS. The nitric oxide (NO) donor spermine NONOate derepressed the Fur regulon that controls secondary lines of resistance against organic acids. Despite inducing a Fur-mediated adaptive response, acidified RNS largely repressed oral virulence as demonstrated by the fact that Salmonella bacteria exposed to NO donors during mildly acidic conditions were shed in low amounts in feces and exhibited ameliorated oral virulence. NO prevented Salmonella from mounting a de novo ATR, but was unable to suppress an already functional protective response, suggesting that RNS target regulatory cascades but not their effectors. Transcriptional and translational analyses revealed that the PhoPQ signaling cascade is a critical ATR target of NO in rapidly growing Salmonella. Inhibition of PhoPQ signaling appears to contribute to most of the NO-mediated abrogation of the ATR in log phase bacteria, because the augmented acid sensitivity of phoQ-deficient Salmonella was not further enhanced after RNS treatment.
Conclusions/significance: Since PhoPQ-regulated acid resistance is widespread in enteric pathogens, the RNS-mediated inhibition of the Salmonella ATR described herein may represent a common component of innate host defenses.
Conflict of interest statement
Figures
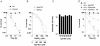

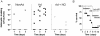

Similar articles
-
Salmonella and Reactive Oxygen Species: A Love-Hate Relationship.J Innate Immun. 2019;11(3):216-226. doi: 10.1159/000496370. Epub 2019 Apr 3. J Innate Immun. 2019. PMID: 30943492 Free PMC article. Review.
-
Magnesium homeostasis protects Salmonella against nitrooxidative stress.Sci Rep. 2017 Nov 8;7(1):15083. doi: 10.1038/s41598-017-15445-y. Sci Rep. 2017. PMID: 29118452 Free PMC article.
-
Nitric oxide and salmonella pathogenesis.Front Microbiol. 2011 Apr 20;2:84. doi: 10.3389/fmicb.2011.00084. eCollection 2011. Front Microbiol. 2011. PMID: 21833325 Free PMC article.
-
Role of the acid tolerance response in virulence of Salmonella typhimurium.Infect Immun. 1996 Apr;64(4):1085-92. doi: 10.1128/iai.64.4.1085-1092.1996. Infect Immun. 1996. PMID: 8606063 Free PMC article.
-
Low pH adaptation and the acid tolerance response of Salmonella typhimurium.Crit Rev Microbiol. 1995;21(4):215-37. doi: 10.3109/10408419509113541. Crit Rev Microbiol. 1995. PMID: 8688153 Review.
Cited by
-
On the difficult evolutionary transition from the free-living lifestyle to obligate symbiosis.PLoS One. 2020 Jul 30;15(7):e0235811. doi: 10.1371/journal.pone.0235811. eCollection 2020. PLoS One. 2020. PMID: 32730262 Free PMC article.
-
Travelers' diarrhea: an update on susceptibility, prevention, and treatment.Curr Gastroenterol Rep. 2008 Oct;10(5):473-9. doi: 10.1007/s11894-008-0087-7. Curr Gastroenterol Rep. 2008. PMID: 18799122
-
Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria.Front Cell Infect Microbiol. 2013 Oct 2;3:59. doi: 10.3389/fcimb.2013.00059. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24106689 Free PMC article. Review.
-
Use of Hybrid Data-Dependent and -Independent Acquisition Spectral Libraries Empowers Dual-Proteome Profiling.J Proteome Res. 2021 Feb 5;20(2):1165-1177. doi: 10.1021/acs.jproteome.0c00350. Epub 2021 Jan 19. J Proteome Res. 2021. PMID: 33467856 Free PMC article.
-
Codependent and independent effects of nitric oxide-mediated suppression of PhoPQ and Salmonella pathogenicity island 2 on intracellular Salmonella enterica serovar typhimurium survival.Infect Immun. 2009 Nov;77(11):5107-15. doi: 10.1128/IAI.00759-09. Epub 2009 Sep 8. Infect Immun. 2009. PMID: 19737903 Free PMC article.
References
-
- Cook GC. Infective gastroenteritis and its relationship to reduced gastric acidity. Scand J Gastroenterol Suppl. 1985;111:17–23. - PubMed
-
- Buchin PJ, Andriole VT, Spiro HM. Salmonella infections and hypochlorhydria. J Clin Gastroenterol. 1980;2:133–138. - PubMed
-
- Holt P. Severe salmonella infection in patients with reduced gastric acidity. Practitioner. 1985;229:1027–1030. - PubMed
-
- Stockbruegger RW. Bacterial overgrowth as a consequence of reduced gastric acidity. Scand J Gastroenterol Suppl. 1985;111:7–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

