A novel calcium-binding protein is associated with tau proteins in tauopathy
- PMID: 18346207
- PMCID: PMC3696493
- DOI: 10.1111/j.1471-4159.2008.05339.x
A novel calcium-binding protein is associated with tau proteins in tauopathy
Abstract
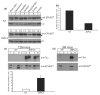
Tauopathies are a group of neurological disorders characterized by the presence of intraneuronal hyperphosphorylated and filamentous tau. Mutations in the tau gene have been found in kindred with tauopathy. The expression of the human tau mutant in transgenic mice induced neurodegeneration, indicating that tau plays a central pathological role. However, the molecular mechanism leading to tau-mediated neurodegeneration is poorly understood. To gain insights into the role that tau plays in neurodegeneration, human tau proteins were immunoprecipitated from brain lysates of the tauopathy mouse model JNPL3, which develops neurodegeneration in age-dependent manner. In the present work, a novel EF-hand domain-containing protein was found associated with tau proteins in brain lysate of 12-month-old JNPL3 mice. The association between tau proteins and the novel identified protein appears to be induced by the neurodegeneration process as these two proteins were not found associated in young JNPL3 mice. Consistently, the novel protein co-purified with the pathological sarkosyl insoluble tau in terminally ill JNPL3 mice. Calcium-binding assays demonstrated that this protein binds calcium effectively. Finally, the association between tau and the novel calcium-binding protein is conserved in human and enriched in Alzheimer's disease brain. Taken together, the identification of a novel calcium-binding protein associated with tau protein in terminally ill tauopathy mouse model and its confirmation in human brain lysate suggests that this association may play an important physiological and/or pathological role.
Figures

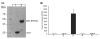
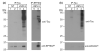

Similar articles
-
EFhd2 is a novel amyloid protein associated with pathological tau in Alzheimer's disease.J Neurochem. 2013 Jun;125(6):921-31. doi: 10.1111/jnc.12155. Epub 2013 Feb 14. J Neurochem. 2013. PMID: 23331044 Free PMC article.
-
Increase in tau tyrosine phosphorylation correlates with the formation of tau aggregates.Brain Res Mol Brain Res. 2005 Aug 18;138(2):135-44. doi: 10.1016/j.molbrainres.2005.04.015. Brain Res Mol Brain Res. 2005. PMID: 15913839 Free PMC article.
-
Amphiphysin-1 protein level changes associated with tau-mediated neurodegeneration.Neuroreport. 2012 Nov 14;23(16):942-6. doi: 10.1097/WNR.0b013e32835982ce. Neuroreport. 2012. PMID: 22975846 Free PMC article.
-
Tau Seeding Mouse Models with Patient Brain-Derived Aggregates.Int J Mol Sci. 2021 Jun 7;22(11):6132. doi: 10.3390/ijms22116132. Int J Mol Sci. 2021. PMID: 34200180 Free PMC article. Review.
-
Analysis of tauopathies with transgenic mice.Trends Mol Med. 2001 Oct;7(10):467-70. doi: 10.1016/s1471-4914(01)02123-2. Trends Mol Med. 2001. PMID: 11597522 Review.
Cited by
-
EF Hand Domain Family Member D2 Is Required for T Cell Cytotoxicity.J Immunol. 2018 Nov 1;201(9):2824-2831. doi: 10.4049/jimmunol.1800839. Epub 2018 Oct 1. J Immunol. 2018. PMID: 30275048 Free PMC article.
-
EFhd2 Affects Tau Liquid-Liquid Phase Separation.Front Neurosci. 2019 Aug 13;13:845. doi: 10.3389/fnins.2019.00845. eCollection 2019. Front Neurosci. 2019. PMID: 31456657 Free PMC article.
-
EFhd2 is a novel amyloid protein associated with pathological tau in Alzheimer's disease.J Neurochem. 2013 Jun;125(6):921-31. doi: 10.1111/jnc.12155. Epub 2013 Feb 14. J Neurochem. 2013. PMID: 23331044 Free PMC article.
-
Maintenance of synaptic stability requires calcium-independent phospholipase A₂ activity.Neural Plast. 2012;2012:569149. doi: 10.1155/2012/569149. Epub 2012 May 20. Neural Plast. 2012. PMID: 22685677 Free PMC article. Review.
-
Abundance of gap junctions at glutamatergic mixed synapses in adult Mosquitofish spinal cord neurons.Front Neural Circuits. 2014 Jun 26;8:66. doi: 10.3389/fncir.2014.00066. eCollection 2014. Front Neural Circuits. 2014. PMID: 25018700 Free PMC article.
References
-
- Avramidou A, Kroczek C, Lang C, Schuh W, Jäck H-M, Mielenz D. The novel adaptor protein Swiprosin 1 enhances BCR signals and contributes to BCR-induced apoptosis. Cell Death Differ. 2007;14:1936–1947. - PubMed
-
- Ballatore C, Lee VM-Y, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 2007;8:663–672. - PubMed
-
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH. Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron. 2003;40:471–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

