Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers
- PMID: 18333863
- PMCID: PMC2311406
- DOI: 10.1111/j.1365-2125.2008.03133.x
Effects of CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers
Abstract
Aims: To evaluate the influence of cytochrome P450 (CYP) 3A4 inhibitors on the clinical pharmacokinetics of maraviroc, a novel CCR5 antagonist.
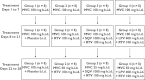
Methods: Four open-label, randomized, placebo-controlled studies were conducted in healthy subjects to assess the effect of separate and distinct combinations of CYP3A4 inhibitors on the steady-state pharmacokinetics of maraviroc. Study 1 was a two-way crossover study investigating the influence of saquinavir (SQV; 1200 mg t.i.d.) and ketoconazole (400 mg q.d.) on the pharmacokinetics of maraviroc (100 mg b.i.d.). All subjects received maraviroc for 7 days in both study periods. Cohort 1 subjects also received SQV or placebo and cohort 2 subjects also received ketoconazole or placebo. Study 2 was a parallel-group study including four treatment groups investigating the effects of ritonavir-boosted lopinavir (LPV/r; 400 mg/100 mg b.i.d.), ritonavir-boosted saquinavir (SQV/r; 1000 mg/100 mg b.i.d.), and low-dose ritonavir (RTV; 100 mg b.i.d.) on the steady-state pharmacokinetics of maraviroc (100 mg b.i.d.), and exploring whether maraviroc dose adjustment can compensate for interaction effects. Treatment lasted 28 days and comprised three distinct phases: (i) maraviroc alone on days 1-7; (ii) maraviroc + interactant on days 8-21; and (iii) maraviroc (adjusted dose) + interactant on days 22-28. Study 3 was a two-way crossover study investigating the effects of atazanavir (ATZ; 400 mg q.d.) and ritonavir-boosted atazanavir (ATZ/r; 300 mg/100 mg b.i.d.) on the pharmacokinetics of maraviroc (300 mg b.i.d.). All subjects received maraviroc on days 1-14 of both study periods. Subjects also received ATZ on days 1-7 and ATZ/r on days 8-14 of one treatment period, and placebo on days 1-14 of the other treatment period. Study 4 was a two-way crossover study investigating the effects of ritonavir-boosted tipranavir (TPV/r; 500 mg/200 mg b.i.d.) on the pharmacokinetics of maraviroc (150 mg b.i.d.). Subjects received maraviroc plus TPV/r or placebo on days 1-8.
Results: All of the drugs/drug combinations tested (except for TPV/r) increased maraviroc exposure, albeit to different degrees of magnitude. SQV/r caused the largest increase in maraviroc exposure (8.3-fold increase in AUC(tau)), whereas RTV caused the smallest increase in maraviroc exposure (2.6-fold increase in AUC(tau)). Downward adjustment of the maraviroc dose in study 2 during co-administration of HIV protease inhibitors was able to compensate for the interactions. TPV/r had no clinically relevant effect on maraviroc exposure at steady state. There were no treatment-related serious adverse events or discontinuations due to adverse events in any of the studies, and most adverse events were mild or moderate in severity and resolved without intervention.
Conclusions: Potent CYP3A4 inhibitors, including ketoconazole and protease inhibitors (except TPV/r), increase maraviroc exposure. Downward adjustment of the maraviroc dose during co-administration with protease inhibitors can compensate for the interaction. TPV/r does not affect the steady-state pharmacokinetics of maraviroc, and hence no dose adjustment would be warranted.
Figures
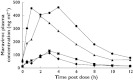
 ); Day 7 MVC + Saquinavir (▴); Day 7 MVC + Placebo (K) (▪); Day 7 MVC + Placebo (SQV) (♦)
); Day 7 MVC + Saquinavir (▴); Day 7 MVC + Placebo (K) (▪); Day 7 MVC + Placebo (SQV) (♦)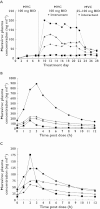
 )
)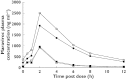
 ); Day 7 MVC + Placebo (▪); Day 14 MVC + ATZ/r (○); Day 14 MVC + Placebo (□)
); Day 7 MVC + Placebo (▪); Day 14 MVC + ATZ/r (○); Day 14 MVC + Placebo (□)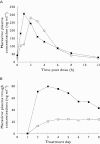
Similar articles
-
Effects of CYP3A4 inducers with and without CYP3A4 inhibitors on the pharmacokinetics of maraviroc in healthy volunteers.Br J Clin Pharmacol. 2008 Apr;65 Suppl 1(Suppl 1):38-46. doi: 10.1111/j.1365-2125.2008.03134.x. Br J Clin Pharmacol. 2008. PMID: 18333864 Free PMC article. Clinical Trial.
-
Maraviroc: in vitro assessment of drug-drug interaction potential.Br J Clin Pharmacol. 2008 Oct;66(4):498-507. doi: 10.1111/j.1365-2125.2008.03198.x. Epub 2008 Apr 10. Br J Clin Pharmacol. 2008. PMID: 18647303 Free PMC article.
-
Drug-drug interaction study of ketoconazole and ritonavir-boosted saquinavir.Antimicrob Agents Chemother. 2009 Feb;53(2):609-14. doi: 10.1128/AAC.00769-08. Epub 2008 Nov 17. Antimicrob Agents Chemother. 2009. PMID: 19015329 Free PMC article. Clinical Trial.
-
CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions--review of the literature.Eur J Med Res. 2007 Oct 15;12(9):409-17. Eur J Med Res. 2007. PMID: 17933722 Review.
-
Clinical pharmacokinetics and summary of efficacy and tolerability of atazanavir.Clin Pharmacokinet. 2005;44(10):1035-50. doi: 10.2165/00003088-200544100-00003. Clin Pharmacokinet. 2005. PMID: 16176117 Review.
Cited by
-
Targeted therapies to treat non-AIDS-defining cancers in patients with HIV on HAART therapy: treatment considerations and research outlook.Curr Opin Oncol. 2009 Sep;21(5):445-54. doi: 10.1097/CCO.0b013e32832f3e04. Curr Opin Oncol. 2009. PMID: 19606034 Free PMC article. Review.
-
A literature review of liver function test elevations in rifampin drug-drug interaction studies.Clin Transl Sci. 2022 Jul;15(7):1561-1580. doi: 10.1111/cts.13281. Epub 2022 May 9. Clin Transl Sci. 2022. PMID: 35470578 Free PMC article. Review.
-
Pharmacokinetic interaction between maraviroc and fosamprenavir-ritonavir: an open-label, fixed-sequence study in healthy subjects.Antimicrob Agents Chemother. 2013 Dec;57(12):6158-64. doi: 10.1128/AAC.01098-13. Epub 2013 Sep 30. Antimicrob Agents Chemother. 2013. PMID: 24080663 Free PMC article. Clinical Trial.
-
Pharmacokinetics of Antiretroviral Drugs in Older People Living with HIV: A Systematic Review.Clin Pharmacokinet. 2023 Sep;62(9):1219-1230. doi: 10.1007/s40262-023-01291-x. Epub 2023 Aug 10. Clin Pharmacokinet. 2023. PMID: 37561283
-
Prediction of drug-drug interactions between various antidepressants and efavirenz or boosted protease inhibitors using a physiologically based pharmacokinetic modelling approach.Clin Pharmacokinet. 2013 Jul;52(7):583-92. doi: 10.1007/s40262-013-0056-7. Clin Pharmacokinet. 2013. PMID: 23479398
References
-
- Choo V. Combination superior to zidovudine in Delta trial. Lancet. 1995;346:895. - PubMed
-
- Collier AC, Coombs RW, Schoenfeld DA, Bassett RL, Timpone J, Baruch A, Jones M, Facey K, Whitacre C, McAuliffe VJ, Friedman HM, Merigan TC, Reichman RC, Hooper C, Corey L. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clinical Trials Group. N Engl J Med. 1996;334:1011–7. - PubMed
-
- Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, Carpenter CC, Fishl MA, Gatell JM, Gazzard BG, Jacobsen DM, Katzenstein DA, Montaner JS, Richman DD, Schooley RT, Thompson MA, Vella S, Volberding PA. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA. 2004;292:251–65. - PubMed
-
- Rusconi S, Scozzafava A, Mastrolorenzo A, Supuran CT. New advances in HIV entry inhibitors development. Curr Drug Targets Infect Disord. 2004;4:339–55. - PubMed
-
- Tremblay C. Effects of HIV-1 entry inhibitors in combination. Curr Pharm Des. 2004;10:1861–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

